03_Worked_Examples
... (c) The reactants box contains four O2 and eight NO. Thus, the molecular ratio is one O2 for each two NO, as required by the balanced equation. The products box contains eight NO 2, which means the number of NO2 product molecules equals the number of NO reactant molecules, as the balanced equation r ...
... (c) The reactants box contains four O2 and eight NO. Thus, the molecular ratio is one O2 for each two NO, as required by the balanced equation. The products box contains eight NO 2, which means the number of NO2 product molecules equals the number of NO reactant molecules, as the balanced equation r ...
Structure of the Atom
... Third orbit or M-shell = 1 electron Or, we can write distribution of electrons in a sodium atom as 2, 8, 1. Question 2: If K and L shells of an atom are full, then what would be the total number of electrons in the atom? Answer: The maximum number of electrons that can occupy K and L-shells of an at ...
... Third orbit or M-shell = 1 electron Or, we can write distribution of electrons in a sodium atom as 2, 8, 1. Question 2: If K and L shells of an atom are full, then what would be the total number of electrons in the atom? Answer: The maximum number of electrons that can occupy K and L-shells of an at ...
Atomic Structure Practice Test
... ____ 10. The smallest unit of an element that can exist either alone or in combination with other such particles of the same or different elements is the a. electron. b. proton. c. neutron. d. atom. ____ 11. The atomic number of oxygen, 8, indicates that there are eight a. protons in the nucleus. c. ...
... ____ 10. The smallest unit of an element that can exist either alone or in combination with other such particles of the same or different elements is the a. electron. b. proton. c. neutron. d. atom. ____ 11. The atomic number of oxygen, 8, indicates that there are eight a. protons in the nucleus. c. ...
CHEM A Midterm Review
... Learning Targets 1.1, 1.5 and 1.12 will be assessed on quizzes, but not the unit test. 1.1 I can write the names and symbols of the elements in columns 1A – 4A on the periodic table. 1.5 I can write the names and symbols of the elements in columns 5A- 8A on the periodic table. 1.12 I can write the n ...
... Learning Targets 1.1, 1.5 and 1.12 will be assessed on quizzes, but not the unit test. 1.1 I can write the names and symbols of the elements in columns 1A – 4A on the periodic table. 1.5 I can write the names and symbols of the elements in columns 5A- 8A on the periodic table. 1.12 I can write the n ...
Mole - My CCSD
... 1. Find the empirical formula. 2. Find the empirical formula mass. 3. Divide the molecular mass by the empirical mass. 4. Multiply each subscript by the ...
... 1. Find the empirical formula. 2. Find the empirical formula mass. 3. Divide the molecular mass by the empirical mass. 4. Multiply each subscript by the ...
Isomers and Isomerism Isomers
... Isomers are different compounds with the same molecular formula. They have the same numbers of same kinds of atoms, but the differ in the way that those atoms are attached to one another, or how they are oriented in space,(Greek: isos= equal; meros=part) Isomerism The presence of two or more compo ...
... Isomers are different compounds with the same molecular formula. They have the same numbers of same kinds of atoms, but the differ in the way that those atoms are attached to one another, or how they are oriented in space,(Greek: isos= equal; meros=part) Isomerism The presence of two or more compo ...
How to balance chemical equations.
... •When pieces of matter come together or break apart, it is called a reaction. •The law of conservation of matter states that matter can not be created or destroyed. •In any reaction, you must have the same amount of each part before and after the reaction happens. •We show how reactions become balan ...
... •When pieces of matter come together or break apart, it is called a reaction. •The law of conservation of matter states that matter can not be created or destroyed. •In any reaction, you must have the same amount of each part before and after the reaction happens. •We show how reactions become balan ...
85 Q.1 A substance X melts at 1600oC. Its does
... 35 Br and 35 Br . The relative atomic mass of bromine is 79.9. Which of the following statements is/are correct? (1) The relative abundance of each isotopic form is about the same. (2) The two isotopes have different numbers of protons. (3) The two isotopes have different numbers of neutrons. ...
... 35 Br and 35 Br . The relative atomic mass of bromine is 79.9. Which of the following statements is/are correct? (1) The relative abundance of each isotopic form is about the same. (2) The two isotopes have different numbers of protons. (3) The two isotopes have different numbers of neutrons. ...
Chemistry Curriculum Map - Belle Vernon Area School District
... the periodic table to its electron configuration and compare its reactivity to the reactivity of other elements in the table. Anchor: CHEM.A.2.2 – Describe the behavior of electrons in atoms. Eligible Content: CHEM.A.2.2.2 – Predict characteristics of an atom or an ion based on its location on the p ...
... the periodic table to its electron configuration and compare its reactivity to the reactivity of other elements in the table. Anchor: CHEM.A.2.2 – Describe the behavior of electrons in atoms. Eligible Content: CHEM.A.2.2.2 – Predict characteristics of an atom or an ion based on its location on the p ...
BERKELEY HEIGHTS PUBLIC SCHOOLS
... technology in developing the models of Dalton, Thomson, Rutherford and Bohr. (5.2 B/1-3; 5.6 A/1; 5.6 A/8; 8.2 A/3) 15. Apply the concepts of radioisotopes, fusion, fission and nuclear decay to understand a half-life, including how to predict the age of a fossil and determine nuclear waste hazards. ...
... technology in developing the models of Dalton, Thomson, Rutherford and Bohr. (5.2 B/1-3; 5.6 A/1; 5.6 A/8; 8.2 A/3) 15. Apply the concepts of radioisotopes, fusion, fission and nuclear decay to understand a half-life, including how to predict the age of a fossil and determine nuclear waste hazards. ...
Problem Set 3_Chem165_Sp2014
... (b) In this question we’ll look mostly at the organic chemistry; maybe in the next problem set we’ll look at the biochemistry. The chemistry is nicely summarized in Figure 1A (the top of Figure 1). Does cleavage of the P–O bond in the compound labeled IPP seem reasonable to you? Does it make a relat ...
... (b) In this question we’ll look mostly at the organic chemistry; maybe in the next problem set we’ll look at the biochemistry. The chemistry is nicely summarized in Figure 1A (the top of Figure 1). Does cleavage of the P–O bond in the compound labeled IPP seem reasonable to you? Does it make a relat ...
AP Biology
... 2. For each of the below listed properties of water – briefly define the property and then explain how water’s polar nature and polar covalent bonds contribute to the water special property. Include an example in nature of each property also. ...
... 2. For each of the below listed properties of water – briefly define the property and then explain how water’s polar nature and polar covalent bonds contribute to the water special property. Include an example in nature of each property also. ...
Pre-AP Chemistry - Simple Rules for Electron Exchange Simple
... cases, we must do some electron bookkeeping. Oxidation numbers are the basis for electron bookkeeping. Tracking how they change as each chemical species goes from reactants to products helps us keep track of which species loses and which species gains electrons. You will note that oxidation numbers ...
... cases, we must do some electron bookkeeping. Oxidation numbers are the basis for electron bookkeeping. Tracking how they change as each chemical species goes from reactants to products helps us keep track of which species loses and which species gains electrons. You will note that oxidation numbers ...
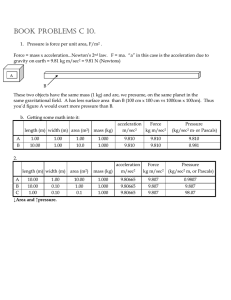
book problems c 10.
... At around this time, Gay-Lussac was studying the chemical reactions of gases, and found that the ratios of volumes of the reacting gases were small integer numbers. This provided a more logical method of assigning atomic weights. Gay-Lussac did not carry through the full implications of his work. Ho ...
... At around this time, Gay-Lussac was studying the chemical reactions of gases, and found that the ratios of volumes of the reacting gases were small integer numbers. This provided a more logical method of assigning atomic weights. Gay-Lussac did not carry through the full implications of his work. Ho ...
Introductory Chemistry, 2nd Edition Nivaldo Tro - Tutor
... Metals are always positive ions, nonmetals are negative ions For many main group metals, the charge = the group number For nonmetals, the charge = the group number - 8 Tro's Introductory Chemistry, Chapter 4 ...
... Metals are always positive ions, nonmetals are negative ions For many main group metals, the charge = the group number For nonmetals, the charge = the group number - 8 Tro's Introductory Chemistry, Chapter 4 ...
TOPIC 7. CHEMICAL CALCULATIONS I
... In Topic 2, atoms were described as ranging from the simplest atom, H, containing a single proton and usually no neutrons in its nucleus with one electron orbiting outside that nucleus, through to very large atoms such as uranium for example which contains 92 protons and even more neutrons in its nu ...
... In Topic 2, atoms were described as ranging from the simplest atom, H, containing a single proton and usually no neutrons in its nucleus with one electron orbiting outside that nucleus, through to very large atoms such as uranium for example which contains 92 protons and even more neutrons in its nu ...
Igcse chemistry lesson 2
... reactions studied in this specification 1.19 use the state symbols (s), (l), (g) and (aq) in chemical equations to represent solids, liquids, gases and aqueous solutions respectively 1.20 understand how the formulae of simple compounds can be obtained experimentally, including metal oxides, water an ...
... reactions studied in this specification 1.19 use the state symbols (s), (l), (g) and (aq) in chemical equations to represent solids, liquids, gases and aqueous solutions respectively 1.20 understand how the formulae of simple compounds can be obtained experimentally, including metal oxides, water an ...
2002 local exam - Virginia Section
... The answers for questions 4 through 7 follow. Select the lettered choice that best fits the statement for each question and fill in the corresponding block on the answer sheet. You may use a choice more than once, once, or not at all. (A) density (B) equilibrium constant (C) freezing point (D) molar ...
... The answers for questions 4 through 7 follow. Select the lettered choice that best fits the statement for each question and fill in the corresponding block on the answer sheet. You may use a choice more than once, once, or not at all. (A) density (B) equilibrium constant (C) freezing point (D) molar ...
docx - California Science Teacher
... elements relates to atomic structure. As a basis for understanding this concept: a. Students know how to relate the position of an element in the periodic table to its atomic number and atomic mass. b. Students know how to use the periodic table to identify metals, semimetals, nonmetals, and halogen ...
... elements relates to atomic structure. As a basis for understanding this concept: a. Students know how to relate the position of an element in the periodic table to its atomic number and atomic mass. b. Students know how to use the periodic table to identify metals, semimetals, nonmetals, and halogen ...
69. (M) Each atom of F contains 9 protons (1.0073 u each), 10
... mass of Kr is 83.80, we can try different values for 84Kr abundance and figure out which gives us the closest value to the given weighted-average isotopic mass: Weighted-Average Percent Isotopic Mass Abundance 84Kr ...
... mass of Kr is 83.80, we can try different values for 84Kr abundance and figure out which gives us the closest value to the given weighted-average isotopic mass: Weighted-Average Percent Isotopic Mass Abundance 84Kr ...
LEGGETT--AP CHEMISTRY * MINIMAL FINAL REVIEW
... compound, and find that it produces 1.481 g of CO2 and 0.303 g of H2O, determine the empirical formula of the compound. (CH) 16. Aluminum bromide is a valuable laboratory chemical. If you use 25.0 mL of liquid bromine (D = 3.1023 g/mL) and excess aluminum metal, what is the maximum theoretical yield ...
... compound, and find that it produces 1.481 g of CO2 and 0.303 g of H2O, determine the empirical formula of the compound. (CH) 16. Aluminum bromide is a valuable laboratory chemical. If you use 25.0 mL of liquid bromine (D = 3.1023 g/mL) and excess aluminum metal, what is the maximum theoretical yield ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.