WEEKLIES ISSUE
... shaped like hooks so they would hold together and atoms of water were slippery, tiny, and smooth so they would flow around each-other. Though this idea of atoms is quite ridiculous by today’s standards, it was the first atomic model to exist—it was also relatively unquestioned for over 2000 years. ...
... shaped like hooks so they would hold together and atoms of water were slippery, tiny, and smooth so they would flow around each-other. Though this idea of atoms is quite ridiculous by today’s standards, it was the first atomic model to exist—it was also relatively unquestioned for over 2000 years. ...
Chapter 03 Atomic Theory
... He asked: Could matter be divided into smaller and smaller pieces forever, or was there a limit to the number of times a piece of matter could be divided? ...
... He asked: Could matter be divided into smaller and smaller pieces forever, or was there a limit to the number of times a piece of matter could be divided? ...
Document
... (E) none of the above 47. Lanthanide or rare earth elements have atoms or ions with partially filled: (A) s subshells (B) p subshells (C) d subshells (D) f subshells (E) g subshells 48. Which of the following liquids would make a good solvent for iodine, I2? (A) HCl (B) H2O (C) CH3OH (D) NH3 (E) CS ...
... (E) none of the above 47. Lanthanide or rare earth elements have atoms or ions with partially filled: (A) s subshells (B) p subshells (C) d subshells (D) f subshells (E) g subshells 48. Which of the following liquids would make a good solvent for iodine, I2? (A) HCl (B) H2O (C) CH3OH (D) NH3 (E) CS ...
Chapter 12 - MrsHenrikssoniClassroom
... • Atoms are always moving and they form different materials by joining together. ...
... • Atoms are always moving and they form different materials by joining together. ...
Atomic Mass
... Caveats of Dalton’s Atomic Theory 1. All elements are composed of tiny indivisible particles called atoms. Atoms are not indivisible – they are made of subatomic particles 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. Every a ...
... Caveats of Dalton’s Atomic Theory 1. All elements are composed of tiny indivisible particles called atoms. Atoms are not indivisible – they are made of subatomic particles 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. Every a ...
Elements Combine to Form Compounds
... Compounds ( 2nd type of Pure Substance) Compound: a pure substance made up of more than ...
... Compounds ( 2nd type of Pure Substance) Compound: a pure substance made up of more than ...
Naming Compounds
... Compounds ( 2nd type of Pure Substance) Compound: a pure substance made up of more than ...
... Compounds ( 2nd type of Pure Substance) Compound: a pure substance made up of more than ...
Earth Materials
... -Diamond and graphite are both made of carbon (C), but why is one the hardest substance on Earth and the other very soft ? ...
... -Diamond and graphite are both made of carbon (C), but why is one the hardest substance on Earth and the other very soft ? ...
MORE ABOUT PROTONS, NEUTRONS AND ELECTRONS
... Using the periodic table again, we can see what makes elements of different atoms unique - it is not the mass (note that cobalt and nickel both have the same Ar, rather it is the number of protons that they have. Atoms are arranged in the periodic table according to the number of protons present. Th ...
... Using the periodic table again, we can see what makes elements of different atoms unique - it is not the mass (note that cobalt and nickel both have the same Ar, rather it is the number of protons that they have. Atoms are arranged in the periodic table according to the number of protons present. Th ...
Energy Atoms and Elements Practice Problems
... B) consist of large particles with very little empty space. C) absorb all -particles that are aimed at them. D) deflect all -particles that are aimed at them. E) are mostly empty space, consisting of a dense nucleus surrounded by tiny electrons. ...
... B) consist of large particles with very little empty space. C) absorb all -particles that are aimed at them. D) deflect all -particles that are aimed at them. E) are mostly empty space, consisting of a dense nucleus surrounded by tiny electrons. ...
Chapter 03 Atomic Theory
... He asked: Could matter be divided into smaller and smaller pieces forever, or was there a limit to the number of times a piece of matter could be divided? ...
... He asked: Could matter be divided into smaller and smaller pieces forever, or was there a limit to the number of times a piece of matter could be divided? ...
Chapter 2 Atoms, Molecules and Ions
... 2. The atoms of a given element are identical; the atoms of different elements are different in some fundamental way or ways 3. Chemical compounds are formed when atoms of different elements combine with each other. A given compound always has the same relative numbers and types of atoms 4. Chemical ...
... 2. The atoms of a given element are identical; the atoms of different elements are different in some fundamental way or ways 3. Chemical compounds are formed when atoms of different elements combine with each other. A given compound always has the same relative numbers and types of atoms 4. Chemical ...
History of the atom
... Matter consists of small particles called atoms. All atoms of one particular element are identical and their properties are identical. Atoms are indestructible. In chemical reaction, the atoms rearrange or combine, but are not ...
... Matter consists of small particles called atoms. All atoms of one particular element are identical and their properties are identical. Atoms are indestructible. In chemical reaction, the atoms rearrange or combine, but are not ...
Exam on Matter through Bonding
... (1) from 2nd to 3rd shell (2) from 2nd to 1st shell (3) from 3rd to 2nd shell (4) from 3rd to 1st shell 2. Which particles are found in the nucleus of an atom? (1) electrons, only (2) neutrons, only (3) protons and electrons (4) protons and neutrons 3. What is the total number of valence electrons i ...
... (1) from 2nd to 3rd shell (2) from 2nd to 1st shell (3) from 3rd to 2nd shell (4) from 3rd to 1st shell 2. Which particles are found in the nucleus of an atom? (1) electrons, only (2) neutrons, only (3) protons and electrons (4) protons and neutrons 3. What is the total number of valence electrons i ...
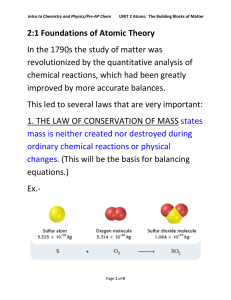
2:1 Foundations of Atomic Theory In the 1790s the study of matter
... neutron are approximately the same size and are 2000 times larger than an electron. If a large football stadium were an atom, this would make the nucleus (including the protons and neutrons) about the size of a marble. The electrons would be similar to dust particles floating about the stadium. This ...
... neutron are approximately the same size and are 2000 times larger than an electron. If a large football stadium were an atom, this would make the nucleus (including the protons and neutrons) about the size of a marble. The electrons would be similar to dust particles floating about the stadium. This ...
Unit 2
... C. atoms are mostly empty space. D. electrons form the nucleus. 33. One part of Dalton's atomic theory that has been modified is the idea that _____ A. atoms cannot be subdivided. B. all matter is composed of atoms. C. atoms of different elements have different properties and masses. D. atoms can co ...
... C. atoms are mostly empty space. D. electrons form the nucleus. 33. One part of Dalton's atomic theory that has been modified is the idea that _____ A. atoms cannot be subdivided. B. all matter is composed of atoms. C. atoms of different elements have different properties and masses. D. atoms can co ...
chemical bonds. - Dr. Gerry Cronin
... • The atoms of a molecule are held together by forces of attraction called chemical bonds. • The likelihood that an atom will form a chemical bond with another atom depends on the number of electrons in its outermost or valence shell. ...
... • The atoms of a molecule are held together by forces of attraction called chemical bonds. • The likelihood that an atom will form a chemical bond with another atom depends on the number of electrons in its outermost or valence shell. ...
WHAT YOU NEED TO KNOW Electron Configurations Explain the
... Recall the reason for the x, y, z, axes. Apply the Pauli exclusion principle, the aufbau principle, and Hund’s rule to write electron configurations using orbital diagrams and electron configuration notation (complete and noble gas). Define valence electrons and apply this knowledge to determi ...
... Recall the reason for the x, y, z, axes. Apply the Pauli exclusion principle, the aufbau principle, and Hund’s rule to write electron configurations using orbital diagrams and electron configuration notation (complete and noble gas). Define valence electrons and apply this knowledge to determi ...
Chemistry Review Answers
... table. Elements within the same group have the same number of electrons in their outer electron shells. Thus, all elements in the same group have similar chemical properties. Groups in the Periodic Table- The elements are arranged in the sequence of their increasing atomic numbers into the periodic ...
... table. Elements within the same group have the same number of electrons in their outer electron shells. Thus, all elements in the same group have similar chemical properties. Groups in the Periodic Table- The elements are arranged in the sequence of their increasing atomic numbers into the periodic ...
Atomic Structure History Dalton-Bohr
... Other philosophers of that time did not agree with his theories. ...
... Other philosophers of that time did not agree with his theories. ...
Atomic Structure History Dem to Bohr
... Other philosophers of that time did not agree with his theories. ...
... Other philosophers of that time did not agree with his theories. ...
"The Atom" Guided Notes
... Bohr Model Bohr discovered that ________________________________ move in orbitals _______________________the nucleus of an atom. Although, we don’t know exactly where electrons are at any given time, we now have a model that helps scientists estimate where electrons are likely to be located at any g ...
... Bohr Model Bohr discovered that ________________________________ move in orbitals _______________________the nucleus of an atom. Although, we don’t know exactly where electrons are at any given time, we now have a model that helps scientists estimate where electrons are likely to be located at any g ...
Radiation
... results in the formation of molecules. Covalently bonded substances are held together with weaker bonds (generally speaking) than ionic substances, which makes them easier to boil, easier to melt, and easier to break back down into the original elements that made up the compound. • In both these rea ...
... results in the formation of molecules. Covalently bonded substances are held together with weaker bonds (generally speaking) than ionic substances, which makes them easier to boil, easier to melt, and easier to break back down into the original elements that made up the compound. • In both these rea ...
Chemistry You Need to Know
... Section 4.1: Development of Atomic Theory Objective: Describe the development of modern atomic theory ...
... Section 4.1: Development of Atomic Theory Objective: Describe the development of modern atomic theory ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.