2 - DanaFrank
... changes that occur when matter interacts in an open and closed container. 8.P.1.1 Classify matter as elements, compounds, or mixtures based on how the atoms are packed together in arrangements. ...
... changes that occur when matter interacts in an open and closed container. 8.P.1.1 Classify matter as elements, compounds, or mixtures based on how the atoms are packed together in arrangements. ...
ATOMIC STRUCTURE
... their inner structure. If atoms are so small that we cannot see them, even with an optical microscope, how can we possibly know anything about their structures? It turns out that we really know quite a lot about the structure of atoms. Although scientists have never examined the interior of an atom ...
... their inner structure. If atoms are so small that we cannot see them, even with an optical microscope, how can we possibly know anything about their structures? It turns out that we really know quite a lot about the structure of atoms. Although scientists have never examined the interior of an atom ...
1 - VCE Chemistry
... 17. What is metal fatigue, how does it occur and what consequences can result from it? 18. List and describe the three methods of heat treatment? 19. How does an atom become an ion? 20. How many protons, neutrons and electrons does (16O8)-2 have? The test will consist of multiple-choice questions an ...
... 17. What is metal fatigue, how does it occur and what consequences can result from it? 18. List and describe the three methods of heat treatment? 19. How does an atom become an ion? 20. How many protons, neutrons and electrons does (16O8)-2 have? The test will consist of multiple-choice questions an ...
C. - Taylor County Schools
... • Chemical reactions involve changes in the electrons surrounding an atom. Nuclear reactions involve changes in the nucleus of an atom. • There are three types of radiation: alpha (charge of 2+), beta (charge of 1–), and gamma (no charge). • The neutron-to-proton ratio of an atom’s nucleus ...
... • Chemical reactions involve changes in the electrons surrounding an atom. Nuclear reactions involve changes in the nucleus of an atom. • There are three types of radiation: alpha (charge of 2+), beta (charge of 1–), and gamma (no charge). • The neutron-to-proton ratio of an atom’s nucleus ...
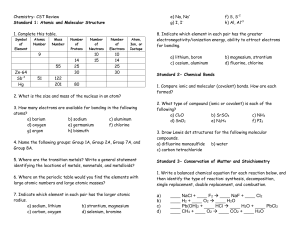
Chemistry- CST Review
... 125 °C. What is the new volume? 10. A 500 mL air sample at a temperature of -50 °C has a pressure of 1.3 atm. What will be the new pressure if the temperature is raised to 102 °C and the volume expands to 700 mL? ...
... 125 °C. What is the new volume? 10. A 500 mL air sample at a temperature of -50 °C has a pressure of 1.3 atm. What will be the new pressure if the temperature is raised to 102 °C and the volume expands to 700 mL? ...
Honors Chemistry
... These are the ones that are on the polyatomic ion sheet and have weird names. The same rules go for the non-metal ( “-ide” or stay the same) The metal can either be the weird name that goes with the charge or The metal can keep its element name and simply take Roman numerals indicating its charge. ...
... These are the ones that are on the polyatomic ion sheet and have weird names. The same rules go for the non-metal ( “-ide” or stay the same) The metal can either be the weird name that goes with the charge or The metal can keep its element name and simply take Roman numerals indicating its charge. ...
How to Balance Chemical Equations
... to be written for all compounds/elements. Make an atom inventory on each side (reactant or product) for all elements involved in the chemical reaction. Select the element that has different number of atoms from one side to another. Find the least common factors for the two numbers. ...
... to be written for all compounds/elements. Make an atom inventory on each side (reactant or product) for all elements involved in the chemical reaction. Select the element that has different number of atoms from one side to another. Find the least common factors for the two numbers. ...
Structure of the atom
... • Bohr’s model worked well in explaining the structure and behavior of simple atoms such as hydrogen. However, it did not explain more complex atoms. • Today’s atomic model is based on the principles of wave mechanics, which involve complex mathematical equations. • According to this model, electron ...
... • Bohr’s model worked well in explaining the structure and behavior of simple atoms such as hydrogen. However, it did not explain more complex atoms. • Today’s atomic model is based on the principles of wave mechanics, which involve complex mathematical equations. • According to this model, electron ...
File
... • When atoms form ions they aim to attain electron shells that are either completely full or completely empty. • If we know the electron configuration of an atom we can usually work out how many electrons it must lose or gain to achieve a noble gas configuration. • This will tell us the charge on it ...
... • When atoms form ions they aim to attain electron shells that are either completely full or completely empty. • If we know the electron configuration of an atom we can usually work out how many electrons it must lose or gain to achieve a noble gas configuration. • This will tell us the charge on it ...
C - Upton-by-Chester High School
... 4) Why would “suck back” have happened if the tube had not been removed at the end? The hot air in the heated test tube would have contracted (1) this would have sucked cold water into the hot test tube, causing it to shatter (1) 5) What happened when bromine water was added to the tube of gas colle ...
... 4) Why would “suck back” have happened if the tube had not been removed at the end? The hot air in the heated test tube would have contracted (1) this would have sucked cold water into the hot test tube, causing it to shatter (1) 5) What happened when bromine water was added to the tube of gas colle ...
Unit 3 – History of Atomic Theory
... As Rutherford’s graduate assistant, Bohr held the planetary model of the atom and incorporated the work of Planck and de Broglie to propose that the electrons must be balanced by the attraction for the nucleus to resist flying off the atom. However a constantly accelerating particle (like the electr ...
... As Rutherford’s graduate assistant, Bohr held the planetary model of the atom and incorporated the work of Planck and de Broglie to propose that the electrons must be balanced by the attraction for the nucleus to resist flying off the atom. However a constantly accelerating particle (like the electr ...
Chapter 4 What are Atoms?
... matter into smaller and smaller fragments, there will be some point at which the pieces cannot be made any smaller. He called these a-tomos which in English would be “un-cuttables”. This proposal was just based on philosophy. It was not until the early 1800s that properties of matter were studied in ...
... matter into smaller and smaller fragments, there will be some point at which the pieces cannot be made any smaller. He called these a-tomos which in English would be “un-cuttables”. This proposal was just based on philosophy. It was not until the early 1800s that properties of matter were studied in ...
Matter and Atoms
... an electron is not a cloud of charge. An electron is one tiny particle. An electron cloud is mostly empty space. At any moment in time, electrons are located at specific points within that area. Electron Energy You have read that electrons are constantly moving around the nucleus in a region called ...
... an electron is not a cloud of charge. An electron is one tiny particle. An electron cloud is mostly empty space. At any moment in time, electrons are located at specific points within that area. Electron Energy You have read that electrons are constantly moving around the nucleus in a region called ...
Atomic Structure File
... charge: electrical charges can be positive or negative. Opposite cancel each other out, so the charge of an atom is the difference between how many positive charges (protons) it has, and how many negative charges (electrons) it has. For example, a chlorine atom with 17 protons (+17) and 18 electrons ...
... charge: electrical charges can be positive or negative. Opposite cancel each other out, so the charge of an atom is the difference between how many positive charges (protons) it has, and how many negative charges (electrons) it has. For example, a chlorine atom with 17 protons (+17) and 18 electrons ...
Physical Science CP Seton Hall Preparatory School Mr. Greene
... Atomic Mass Units (AMU) Isotopes Calculation of the number of neutrons/protons contained in an isotope Ions; cations vs. anions Periodic Table: Period Group Properties of metals, nonmetals, and metalloids Periodic trends; atomic radius, electronegativity, and metallic character Major groups; alkali ...
... Atomic Mass Units (AMU) Isotopes Calculation of the number of neutrons/protons contained in an isotope Ions; cations vs. anions Periodic Table: Period Group Properties of metals, nonmetals, and metalloids Periodic trends; atomic radius, electronegativity, and metallic character Major groups; alkali ...
The Atom - dsapresents.org
... Dalton’s Atomic Theory 1. All elements are composed of tiny indivisible particles called atoms 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. 3. Atoms can physically mix together or can chemically combine in simple whole numbe ...
... Dalton’s Atomic Theory 1. All elements are composed of tiny indivisible particles called atoms 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. 3. Atoms can physically mix together or can chemically combine in simple whole numbe ...
Atoms – Building Blocks of Matter Notes
... J.J. Thomson and Robert Millikan) 1st subatomic particle to be discovered – Thompson was working with electricity and magnetic fields. He was taking various gases and sending an electric current through the gas. When he did this he noticed that a glow was emitted. (What he was doing, he believed, wa ...
... J.J. Thomson and Robert Millikan) 1st subatomic particle to be discovered – Thompson was working with electricity and magnetic fields. He was taking various gases and sending an electric current through the gas. When he did this he noticed that a glow was emitted. (What he was doing, he believed, wa ...
CHEM 1A General Chemistry I (1)
... intended for majors in the natural sciences (chemistry, biochemistry, biology, physics, pre-medicine), mathematics, and engineering. Prerequisite(s): One year of high school chemistry,Chemistry 45 or the equivalent. MATH 60 Intermediate Algebra Upon entering the course, the student should be able to ...
... intended for majors in the natural sciences (chemistry, biochemistry, biology, physics, pre-medicine), mathematics, and engineering. Prerequisite(s): One year of high school chemistry,Chemistry 45 or the equivalent. MATH 60 Intermediate Algebra Upon entering the course, the student should be able to ...
Review Package
... 7) Draw a Bohr-Rutherford/Lewis diagram for the stable ions formed by each of the following atom: a) State the number of electrons gained or lost to form each ion. b) State the ionic charge on each of the ions. c) Name the noble gas that is isoelectric with each of the stable ions. ...
... 7) Draw a Bohr-Rutherford/Lewis diagram for the stable ions formed by each of the following atom: a) State the number of electrons gained or lost to form each ion. b) State the ionic charge on each of the ions. c) Name the noble gas that is isoelectric with each of the stable ions. ...
Atoms
... Which will identify isotopes Example: How many protons, electrons and neutrons are there in an atom of chlorine-37? 37 – 17(atomic # =protons and electrons) =20 neutrons ...
... Which will identify isotopes Example: How many protons, electrons and neutrons are there in an atom of chlorine-37? 37 – 17(atomic # =protons and electrons) =20 neutrons ...
Part II Biochemistry
... Carbohydrates occur in all plants and animals and are essential to life. Through photosynthesis, plants convert atmospheric carbon dioxide to carbohydrates, mainly cellulose, starch, and sugars. Cellulose is the building block of rigid cell walls and woody tissues in plants, whereas starch is ...
... Carbohydrates occur in all plants and animals and are essential to life. Through photosynthesis, plants convert atmospheric carbon dioxide to carbohydrates, mainly cellulose, starch, and sugars. Cellulose is the building block of rigid cell walls and woody tissues in plants, whereas starch is ...
Structure of the Atom
... 3) Atoms combine in whole-number ratios to form compounds 4) In chemical reactions, atoms are combined, separated, or rearranged – but never changed into atoms of another element. ...
... 3) Atoms combine in whole-number ratios to form compounds 4) In chemical reactions, atoms are combined, separated, or rearranged – but never changed into atoms of another element. ...
600 $600
... Which is always true of an endothermic reaction? A. Chemical bonds are broken. B. Energy is released. C. Light is created. D. Energy is absorbed. ...
... Which is always true of an endothermic reaction? A. Chemical bonds are broken. B. Energy is released. C. Light is created. D. Energy is absorbed. ...
Week 9 CCA Test Review
... Why do elements with similar valence level of electrons have similar chemical properties? They will react the same way, because they ...
... Why do elements with similar valence level of electrons have similar chemical properties? They will react the same way, because they ...
the Student Handout
... The Bohr model of the atom followed. In the Bohr model, nucleus was the center but now the electrons “lived” in energy levels. This addition to the Rutherford model helps explain why only cer ...
... The Bohr model of the atom followed. In the Bohr model, nucleus was the center but now the electrons “lived” in energy levels. This addition to the Rutherford model helps explain why only cer ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.