writing and balancing equations
... 2. There must be the same number of each kind of atom on each side of the arrow. This is done by using coefficients (numbers in front of a formula). 3. Do not change formulas to balance atoms!! 4. Follow a trial and error process. Balance each species one at a time. Be prepared to erase!! ...
... 2. There must be the same number of each kind of atom on each side of the arrow. This is done by using coefficients (numbers in front of a formula). 3. Do not change formulas to balance atoms!! 4. Follow a trial and error process. Balance each species one at a time. Be prepared to erase!! ...
Atomic Structure and the Periodic Table
... the metals are on the left and the nonmetals are on the right (hydrogen is a nonmetal but is often put in the middle) the main groups are numbered from 1 to 7 going from left to right, and the last group on the right is Group 0 the block in between Group 2 and Group 3 is where the transition m ...
... the metals are on the left and the nonmetals are on the right (hydrogen is a nonmetal but is often put in the middle) the main groups are numbered from 1 to 7 going from left to right, and the last group on the right is Group 0 the block in between Group 2 and Group 3 is where the transition m ...
Hein and Arena - faculty at Chemeketa
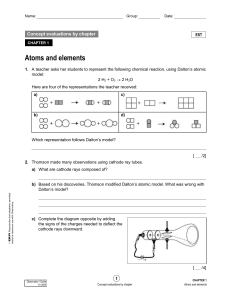
... Dalton’s Atomic Theory 1. Elements are composed of minute indivisible particles called atoms. 2. Atoms of the same element are alike in mass and size. 3. Atoms of different elements have different masses and sizes. Modernunder research has circumstances demonstrated that Atoms special can ...
... Dalton’s Atomic Theory 1. Elements are composed of minute indivisible particles called atoms. 2. Atoms of the same element are alike in mass and size. 3. Atoms of different elements have different masses and sizes. Modernunder research has circumstances demonstrated that Atoms special can ...
The Periodic Table, Atomic Structure, Isotopes, Ions and Nomenclature
... • Thus far, we’ve learned that each element has an exact number of protons. – For example, Hydrogen has only one proton. If you force a second proton onto the atom, you no longer have hydrogen… you now have Helium. • We have also learned that atoms can have variable numbers of ...
... • Thus far, we’ve learned that each element has an exact number of protons. – For example, Hydrogen has only one proton. If you force a second proton onto the atom, you no longer have hydrogen… you now have Helium. • We have also learned that atoms can have variable numbers of ...
Chapter 5
... 2. All atoms of an element are identical and have the same properties. 3. Atoms of different elements combine to form compounds. ...
... 2. All atoms of an element are identical and have the same properties. 3. Atoms of different elements combine to form compounds. ...
Chapter 22-Newest-CD
... • Enantiomers are “optical isomers.” eg. (+) and (-) carvone • Most physical and chemical properties of enantiomers are identical. • Therefore, enantiomers are very difficult to separate eg. Tartaric acid…ask Louis Pasteur. • Enzymes are catalysts that are very specific, acting on only one enantiome ...
... • Enantiomers are “optical isomers.” eg. (+) and (-) carvone • Most physical and chemical properties of enantiomers are identical. • Therefore, enantiomers are very difficult to separate eg. Tartaric acid…ask Louis Pasteur. • Enzymes are catalysts that are very specific, acting on only one enantiome ...
File
... C. Energy is added or removed and temperature stays the same D. None of the above 96. What is specific heat? A. a measure of kinetic energy B. the energy of motion C. how much space something takes up D. the amount of energy required to raise the temperature of a substance 97. Which requires the mos ...
... C. Energy is added or removed and temperature stays the same D. None of the above 96. What is specific heat? A. a measure of kinetic energy B. the energy of motion C. how much space something takes up D. the amount of energy required to raise the temperature of a substance 97. Which requires the mos ...
FIREWORKS EMC summary notes
... Most elements are solid at room temperature, e.g. carbon and copper. The two elements that are liquid at room temperature are bromine and mercury. Some elements are gases at room temperature, e.g. oxygen and hydrogen.* Elements can be classified as metals and non-metals. There are many more metals t ...
... Most elements are solid at room temperature, e.g. carbon and copper. The two elements that are liquid at room temperature are bromine and mercury. Some elements are gases at room temperature, e.g. oxygen and hydrogen.* Elements can be classified as metals and non-metals. There are many more metals t ...
Atomic Structure - Physical Science
... •The nucleus of an atom contains roughly 99.9% of the mass of an atom. •More than 99.9% (that's a popular number) of the atom is "empty space" occupied by moving electrons (which weigh very little). If you could make an atom as big as a football stadium, then its nucleus would still be as small as a ...
... •The nucleus of an atom contains roughly 99.9% of the mass of an atom. •More than 99.9% (that's a popular number) of the atom is "empty space" occupied by moving electrons (which weigh very little). If you could make an atom as big as a football stadium, then its nucleus would still be as small as a ...
Chemistry Midterm Review 2006
... 5. Which of the following compounds contain ionic bonds? H2O, Na2O, CO2, CaS2, SO2, CaCO3. 6. Know the difference between a formula unit and a molecule. 7. What is the difference between a nonpolar covalent bond and a polar covalent bond? 8. What are the properties of a covalent and ionic compound i ...
... 5. Which of the following compounds contain ionic bonds? H2O, Na2O, CO2, CaS2, SO2, CaCO3. 6. Know the difference between a formula unit and a molecule. 7. What is the difference between a nonpolar covalent bond and a polar covalent bond? 8. What are the properties of a covalent and ionic compound i ...
Unit B review - mvhs
... Multiple Choice: Most of the following are actual questions from previous AP Exams. You may work on them alone or with partners, but try to complete them using only a periodic table and calculator, if necessary. These 30 questions should take you about 30 minutes to finish. ...
... Multiple Choice: Most of the following are actual questions from previous AP Exams. You may work on them alone or with partners, but try to complete them using only a periodic table and calculator, if necessary. These 30 questions should take you about 30 minutes to finish. ...
Chapter 14 – Chemical Reactions
... Reactants – the _____________ materials of a chemical _____________ Products – the substances _____________ as a _____________ of a chemical _____________ Coefficient – a _____________ placed in _____________ of a chemical _____________ or _____________ All chemical equations must be balanced. Steps ...
... Reactants – the _____________ materials of a chemical _____________ Products – the substances _____________ as a _____________ of a chemical _____________ Coefficient – a _____________ placed in _____________ of a chemical _____________ or _____________ All chemical equations must be balanced. Steps ...
Chapter 3 Stoichiometry: Chemical Calculations
... Calculating the empirical formula: 1. can be determined from masses of the individual elements or the % composition of the elements in a compound 2. if %’s are given consider the sample to be of 100 grams and so the %’s become the masses in grams: For example: 25.6% = 25.6 g 3. convert the mass of e ...
... Calculating the empirical formula: 1. can be determined from masses of the individual elements or the % composition of the elements in a compound 2. if %’s are given consider the sample to be of 100 grams and so the %’s become the masses in grams: For example: 25.6% = 25.6 g 3. convert the mass of e ...
Unit B: Matter and Chemical Change
... Laws: Describe and summarize what happens in a natural system. Theories: Imaginative ways to explain why something happens in a natural system. Models: Help picture structures or processes that cannot be directly seen. Observations: Thousands of observations must be made before the scientific commun ...
... Laws: Describe and summarize what happens in a natural system. Theories: Imaginative ways to explain why something happens in a natural system. Models: Help picture structures or processes that cannot be directly seen. Observations: Thousands of observations must be made before the scientific commun ...
Unit 10 Test Review
... a. movement of electrons in circular orbits. b. movement of electrons from higher energy states to lower energy states. c. movement of electrons from lower energy states to higher energy states. d. movement of electrons as they fall into the nucleus. 11. How many neutrons are contained in an atom of ...
... a. movement of electrons in circular orbits. b. movement of electrons from higher energy states to lower energy states. c. movement of electrons from lower energy states to higher energy states. d. movement of electrons as they fall into the nucleus. 11. How many neutrons are contained in an atom of ...
Atoms - Learn More Chemistry
... * the proton remains in the nucleus and the electron (now the beta particle) is propelled out of the nucleus at high speed * the mass number for a beta particle is zero because an electron has a very small mass compared with a proton or neutron * beta radiation is approx. 100 times more penetrating ...
... * the proton remains in the nucleus and the electron (now the beta particle) is propelled out of the nucleus at high speed * the mass number for a beta particle is zero because an electron has a very small mass compared with a proton or neutron * beta radiation is approx. 100 times more penetrating ...
5a. Bonding Chemical Bonds Linkage which holds Types of
... place lone pairs around each terminal atom bonded to the central atom to satisfy the octet rule. Any remaining pairs are assigned to the central atom. ...
... place lone pairs around each terminal atom bonded to the central atom to satisfy the octet rule. Any remaining pairs are assigned to the central atom. ...
Cluster Assembled Metal Encapsulated Thin Nanotubes of Silicon
... reflection passing through its center. This is composed of Si24Be2 structure. • In the second diagram the Be atom is placed between successive hexagons and the doped portion is symmetric while the remaining nanotube becomes ...
... reflection passing through its center. This is composed of Si24Be2 structure. • In the second diagram the Be atom is placed between successive hexagons and the doped portion is symmetric while the remaining nanotube becomes ...
Mass Defect (not in book)
... The forces that hold together the protons and neutrons in the nuclei of atoms are much larger than the forces that bind atoms together to form molecules. In fact, the energies associated with nuclear processes are more than a million times those associated with chemical reactions. This fact potentia ...
... The forces that hold together the protons and neutrons in the nuclei of atoms are much larger than the forces that bind atoms together to form molecules. In fact, the energies associated with nuclear processes are more than a million times those associated with chemical reactions. This fact potentia ...
11129_evl_ch1_ste_eleve (3)
... 7. Six different elements are represented below according to the Rutherford-Bohr atomic model. ...
... 7. Six different elements are represented below according to the Rutherford-Bohr atomic model. ...
Name Test Review Chapters 4 and 25 Honors Chemistry 1. Fill in
... sample, how much will be left over after 104 minutes? 23. Mercury -197 is used for kidney scans and has a half-life of 3 days. If the amount of mercury-197 needed for a study is 1.0 gram and the time allowed for shipment is 15 days, how much mercury-197 will need to be ordered? 24. How much strontiu ...
... sample, how much will be left over after 104 minutes? 23. Mercury -197 is used for kidney scans and has a half-life of 3 days. If the amount of mercury-197 needed for a study is 1.0 gram and the time allowed for shipment is 15 days, how much mercury-197 will need to be ordered? 24. How much strontiu ...
4.1Atoms and Isotopes
... Therefore, each atomic number is unique and defines each atom Eg: Oxygen has an atomic number of 8 because it has 8 protons Easily recognized on the periodic table for each element (see ...
... Therefore, each atomic number is unique and defines each atom Eg: Oxygen has an atomic number of 8 because it has 8 protons Easily recognized on the periodic table for each element (see ...
atoms
... – Cations are positive and are formed by elements on the left side of the periodic chart. – Anions are negative and are formed by elements on the right side of the periodic chart. © 2012 Pearson Education, Inc. ...
... – Cations are positive and are formed by elements on the left side of the periodic chart. – Anions are negative and are formed by elements on the right side of the periodic chart. © 2012 Pearson Education, Inc. ...
Back - WasmundScience
... C1-100-The answer is… Which scientist came up with the idea that all matter is made up of tiny pieces of matter and named them atomos? Democritus Back ...
... C1-100-The answer is… Which scientist came up with the idea that all matter is made up of tiny pieces of matter and named them atomos? Democritus Back ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.