Term 1 and 2 Powerpoints
... thinking about our environment and the things that we burn which pollute it. I then thought of where all the statistics we hear about come from, and how the claims are substantiated. How do scientists know exactly what percent our ozone layer has deteriorated, and what percent of our atmosphere is m ...
... thinking about our environment and the things that we burn which pollute it. I then thought of where all the statistics we hear about come from, and how the claims are substantiated. How do scientists know exactly what percent our ozone layer has deteriorated, and what percent of our atmosphere is m ...
atoms and molecules - Mockiesgateacademy
... of the elementary gases consist of homo atomic molecules. For example hydrogen gas consists of two atoms of hydrogen (H2). Similarly, oxygen gas consists of two atoms of oxygen (O2). In accordance with the number of atoms present in these molecules, they are classified as monoatomic, diatomic, triat ...
... of the elementary gases consist of homo atomic molecules. For example hydrogen gas consists of two atoms of hydrogen (H2). Similarly, oxygen gas consists of two atoms of oxygen (O2). In accordance with the number of atoms present in these molecules, they are classified as monoatomic, diatomic, triat ...
Atomic Notation
... -Chemical symbols were proposed in 1817 and were adopted internationally -The atom was recognized as being the smallest part of an element at that time -Atoms are composed of protons (positively charged), electrons (negatively charged), and neutrons (no charge) -Protons and neutrons are found in the ...
... -Chemical symbols were proposed in 1817 and were adopted internationally -The atom was recognized as being the smallest part of an element at that time -Atoms are composed of protons (positively charged), electrons (negatively charged), and neutrons (no charge) -Protons and neutrons are found in the ...
Nothing Lost, Nothing Gained
... One thing you should know about the world is that no matter what happens, it always has the same amount of stuff. Burn something. Break something. Build something out of sand. All of the little parts, or atoms, that make up everything never grow in number and they never shrink in number. Things migh ...
... One thing you should know about the world is that no matter what happens, it always has the same amount of stuff. Burn something. Break something. Build something out of sand. All of the little parts, or atoms, that make up everything never grow in number and they never shrink in number. Things migh ...
File
... 55. Which pair of solutions forms a buffer when equal volumes of each are mixed? A) 0.20 M HCl and 0.20 M NaCl C) 0.20 M HCl and 0.20 M NH3 B) 0.40 M HC2H3O2 and 0.20 M NaOH D) 0.40 M HCl and 0.20 M NH3 56. A student is attempting to standardize a NaOH solution with a 0.500 molar solution of oxalic ...
... 55. Which pair of solutions forms a buffer when equal volumes of each are mixed? A) 0.20 M HCl and 0.20 M NaCl C) 0.20 M HCl and 0.20 M NH3 B) 0.40 M HC2H3O2 and 0.20 M NaOH D) 0.40 M HCl and 0.20 M NH3 56. A student is attempting to standardize a NaOH solution with a 0.500 molar solution of oxalic ...
Balancing Chemical Equations – A Primer
... Done yet...ah, no. Next, we have to consider POLYATOMIC IONS. Say what? These are two elements that SHARE electrons. This is called a COVALENT BOND. For the moment, these are elements located on the right side of the Periodic Table. Thus, two negatively charged elements bonded. How do two negatives ...
... Done yet...ah, no. Next, we have to consider POLYATOMIC IONS. Say what? These are two elements that SHARE electrons. This is called a COVALENT BOND. For the moment, these are elements located on the right side of the Periodic Table. Thus, two negatively charged elements bonded. How do two negatives ...
E/F Physical Science
... 1. Is the following sentence true or false? The new substances formed as a result of a chemical reaction are called products. 2. Circle the letter of each sentence that is correct for the chemical equation: C + O2 → CO2. a. Carbon and oxygen react and form carbon monoxide. b. Carbon and oxygen react ...
... 1. Is the following sentence true or false? The new substances formed as a result of a chemical reaction are called products. 2. Circle the letter of each sentence that is correct for the chemical equation: C + O2 → CO2. a. Carbon and oxygen react and form carbon monoxide. b. Carbon and oxygen react ...
AP Chemistry Summer Assignment 2016
... make the best use of our class time. You need to refresh what you learned in Chemistry Honors so that you’re ready to move forward from there. There will be a quiz on Chapters 1 and 2 and a test on Chapters 3 and 4 during the first few days of school In addition to the actual summer assignment there ...
... make the best use of our class time. You need to refresh what you learned in Chemistry Honors so that you’re ready to move forward from there. There will be a quiz on Chapters 1 and 2 and a test on Chapters 3 and 4 during the first few days of school In addition to the actual summer assignment there ...
Chapter 6 Electronic Structure of Atoms
... shape of the orbital. • Allowed values of l are integers ranging from 0 to n − 1. • We use letter designations to communicate the different values of l and, therefore, the shapes and types of Electronic orbitals. Structure of Atoms © 2009, Prentice-Hall, Inc. ...
... shape of the orbital. • Allowed values of l are integers ranging from 0 to n − 1. • We use letter designations to communicate the different values of l and, therefore, the shapes and types of Electronic orbitals. Structure of Atoms © 2009, Prentice-Hall, Inc. ...
Chapter 6 Electronic Structure of Atoms
... shape of the orbital. • Allowed values of l are integers ranging from 0 to n − 1. • We use letter designations to communicate the different values of l and, therefore, the shapes and types of Electronic orbitals. Structure of Atoms © 2009, Prentice-Hall, Inc. ...
... shape of the orbital. • Allowed values of l are integers ranging from 0 to n − 1. • We use letter designations to communicate the different values of l and, therefore, the shapes and types of Electronic orbitals. Structure of Atoms © 2009, Prentice-Hall, Inc. ...
Atoms - ChemGod.com
... The Atomic Theory of Matter At its lowest level, matter is made up of atoms. The current theory is most directly traceable to John Dalton in the early 1800s. ...
... The Atomic Theory of Matter At its lowest level, matter is made up of atoms. The current theory is most directly traceable to John Dalton in the early 1800s. ...
General Chemistry I - University of Toledo
... 7.1 Describe the difference between an ionic and covalent bond. 7.2 Describe changes in energy that occur as two nuclei approach to form a covalent bond. 7.3 Name a covalent compound given the chemical formula. 7.4 Predict trends in bond length and bond dissociation energy based on bond order and at ...
... 7.1 Describe the difference between an ionic and covalent bond. 7.2 Describe changes in energy that occur as two nuclei approach to form a covalent bond. 7.3 Name a covalent compound given the chemical formula. 7.4 Predict trends in bond length and bond dissociation energy based on bond order and at ...
Hantaro Nagaoka
... the Saturnian Atomic Model which was the idea that in the nucleus there were protons and surrounded by the nucleus were electrons. Author: James Trefil, Atom, Encyclopedia Brittanica http://www.britannica.com/EBchecked/t opic/41549/atom/48356/Discovery-ofradioactivity Date: September 4, 2014 ...
... the Saturnian Atomic Model which was the idea that in the nucleus there were protons and surrounded by the nucleus were electrons. Author: James Trefil, Atom, Encyclopedia Brittanica http://www.britannica.com/EBchecked/t opic/41549/atom/48356/Discovery-ofradioactivity Date: September 4, 2014 ...
sample - Bright Red Publishing
... reactants and products. However, there is no way we can determine the absolute value of the enthalpy of a substance. Only values relative to an arbitrary reference point can be given and for all enthalpy expressions, this reference point is called the standard enthalpy of formation. The standard ent ...
... reactants and products. However, there is no way we can determine the absolute value of the enthalpy of a substance. Only values relative to an arbitrary reference point can be given and for all enthalpy expressions, this reference point is called the standard enthalpy of formation. The standard ent ...
Chapter 13 Electrons in Atoms
... 7. He stated that it is impossible to know the exact position and momentum of an electron in an atom. ...
... 7. He stated that it is impossible to know the exact position and momentum of an electron in an atom. ...
Bohr Model - TeacherWeb
... For each proton that an element has in a neutral atom there is one electron. It is almost as if they were a matched pair that comes to a couples only dance party. Each electron must fit into an electron cloud around the nucleus, but there are only certain places that they can be. They always fill i ...
... For each proton that an element has in a neutral atom there is one electron. It is almost as if they were a matched pair that comes to a couples only dance party. Each electron must fit into an electron cloud around the nucleus, but there are only certain places that they can be. They always fill i ...
Valence Electrons
... In the 1700s, Lavoisier compiled a list of all the known elements of the time. ...
... In the 1700s, Lavoisier compiled a list of all the known elements of the time. ...
Science 9 Unit B 2.0 - Vegreville Composite High
... by using their properties, however, this was difficult because different scientists used different properties • John Dalton first attempted to categorize and order the elements in the early 1800’s. He developed a new system of symbols ...
... by using their properties, however, this was difficult because different scientists used different properties • John Dalton first attempted to categorize and order the elements in the early 1800’s. He developed a new system of symbols ...
Main Group Notes 1
... contain bonds between metals and carbon. These were thus some of the initial examples of organometallic chemistry (one of the most studied branches of inorganic chemistry today). ...
... contain bonds between metals and carbon. These were thus some of the initial examples of organometallic chemistry (one of the most studied branches of inorganic chemistry today). ...
Science 1206 Unit 3 Part 1
... formula H2O and the empirical formula H2O since the atoms are already in the simplest form. Whereas hydrogen peroxide has the molecular formula H2O2 and the empirical formula HO. Polyatomic ion – an ion that consists of two or more different non-metal atoms that are joined by covalent bonds ...
... formula H2O and the empirical formula H2O since the atoms are already in the simplest form. Whereas hydrogen peroxide has the molecular formula H2O2 and the empirical formula HO. Polyatomic ion – an ion that consists of two or more different non-metal atoms that are joined by covalent bonds ...
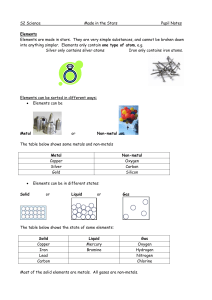
Made in the Stars Notes
... at room temperature except for mercury, which is a liquid. Non-metal solids are usually brittle (they break easily). Non-metals can be solids, liquids or gases at room temperature. Non-metals usually have low melting and boiling points. They are poor conductors of electricity. The exception is graph ...
... at room temperature except for mercury, which is a liquid. Non-metal solids are usually brittle (they break easily). Non-metals can be solids, liquids or gases at room temperature. Non-metals usually have low melting and boiling points. They are poor conductors of electricity. The exception is graph ...
Dating the Earth Power Point
... history of Earth and determine evolutionary pathways. Radioactive dating is an important tool scientists use to do this. To find a radioactive date, the object being dated must contain a radioactive element such as uranium-235 or carbon 14. These elements are decaying by emitting a small part of the ...
... history of Earth and determine evolutionary pathways. Radioactive dating is an important tool scientists use to do this. To find a radioactive date, the object being dated must contain a radioactive element such as uranium-235 or carbon 14. These elements are decaying by emitting a small part of the ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.