CHAP5
... atoms • This is attained by referring to some basic experimental facts that have been gathered since 1900’s (e.g. Rutherford scattering experiment, atomic spectral lines etc.) • In order to build a model that well describes the atoms which are consistent with the experimental facts, we need to take ...
... atoms • This is attained by referring to some basic experimental facts that have been gathered since 1900’s (e.g. Rutherford scattering experiment, atomic spectral lines etc.) • In order to build a model that well describes the atoms which are consistent with the experimental facts, we need to take ...
Unit 2 Review Game
... • The law that states that when two elements combine to form two or more compounds, the mass of one element that combines with a given mass of the other is in ratio of small whole number. ...
... • The law that states that when two elements combine to form two or more compounds, the mass of one element that combines with a given mass of the other is in ratio of small whole number. ...
Atomic mod
... Basic properties of atoms • 1) Atoms are of microscopic size, ~ 10-10 m. Visible light is not enough to resolve (see) the detail structure of an atom as its size is only of the order of 100 nm. • 2) Atoms are stable • 3) Atoms contain negatively charges, electrons, but are electrically neutral. An ...
... Basic properties of atoms • 1) Atoms are of microscopic size, ~ 10-10 m. Visible light is not enough to resolve (see) the detail structure of an atom as its size is only of the order of 100 nm. • 2) Atoms are stable • 3) Atoms contain negatively charges, electrons, but are electrically neutral. An ...
Laws
... • The law that states that when two elements combine to form two or more compounds, the mass of one element that combines with a given mass of the other is in ratio of small whole number. ...
... • The law that states that when two elements combine to form two or more compounds, the mass of one element that combines with a given mass of the other is in ratio of small whole number. ...
Section 2.9 Molar Mass: Counting Atoms by Weighing Them
... called atoms. All atoms of a given element have the same mass and other properties that distinguish them from atoms of other elements Atoms combine in simple whole number ratios to form compounds. Atoms of one element cannot change into atoms of another element. In a chemical reaction, atoms only ch ...
... called atoms. All atoms of a given element have the same mass and other properties that distinguish them from atoms of other elements Atoms combine in simple whole number ratios to form compounds. Atoms of one element cannot change into atoms of another element. In a chemical reaction, atoms only ch ...
NOBLE-GAS CHEMISTRY
... adducts of noble gases to a metal center, NgBe(II)O, where Ng = Ar, Kr, and Xe.31 The case of XeBeO is rather straightforward: a coordinatively unsaturated Be(II) cation exposes its empty (sp) hybrid, and is ready to bind whatever Lewis base you provide. Hence, it will bind a Xe atom with a surprisi ...
... adducts of noble gases to a metal center, NgBe(II)O, where Ng = Ar, Kr, and Xe.31 The case of XeBeO is rather straightforward: a coordinatively unsaturated Be(II) cation exposes its empty (sp) hybrid, and is ready to bind whatever Lewis base you provide. Hence, it will bind a Xe atom with a surprisi ...
t2 – modern atomic theory continued
... 3. Atoms are electrically neutral which means they contain a positive charge that is equal to number of electrons (negative charge) within the atom. IV) ERNEST RUTHERFORD A) The models developed by both Nagaoka and Thomson explained the existing evidence equally well, thus both models were equally v ...
... 3. Atoms are electrically neutral which means they contain a positive charge that is equal to number of electrons (negative charge) within the atom. IV) ERNEST RUTHERFORD A) The models developed by both Nagaoka and Thomson explained the existing evidence equally well, thus both models were equally v ...
Chemistry 20
... m) Copper metal and silver nitrate react to form silver metal and copper (II) nitrate. n) Sodium metal and chlorine react to make sodium chloride. o) Calcium phosphate and sulfuric acid make calcium sulfate and phosphoric acid. 13. Describe the difference between ionic and molecular compounds. You m ...
... m) Copper metal and silver nitrate react to form silver metal and copper (II) nitrate. n) Sodium metal and chlorine react to make sodium chloride. o) Calcium phosphate and sulfuric acid make calcium sulfate and phosphoric acid. 13. Describe the difference between ionic and molecular compounds. You m ...
4 ATOMIC STRUCTURE NOTES __ /__ pts
... 20. Describe Rutherford’s model of the atom, including the location of protons, neutrons, and electrons with respect to the nucleus. How does this model explain the deflections of a beam of alpha particles aimed at a sheet of gold foil? ...
... 20. Describe Rutherford’s model of the atom, including the location of protons, neutrons, and electrons with respect to the nucleus. How does this model explain the deflections of a beam of alpha particles aimed at a sheet of gold foil? ...
pdf.format - San Diego Mesa College
... •varaible shape and volume that fills the container •high compressibility •complete freedom of motion •extreme disorder •flow and diffuse easily •weakest attractive forces •least dense state •exert a pressure easily ...
... •varaible shape and volume that fills the container •high compressibility •complete freedom of motion •extreme disorder •flow and diffuse easily •weakest attractive forces •least dense state •exert a pressure easily ...
Chapter 8: Periodic Properties of the Elements
... when electrons are forced to share space in a filled orbital they are slightly higher in energy, so it is slightly easier to remove one giving O a smaller ionization energy compared to N. ...
... when electrons are forced to share space in a filled orbital they are slightly higher in energy, so it is slightly easier to remove one giving O a smaller ionization energy compared to N. ...
NH3 Cu NH3 NH3 H3N - Wilson`s Disease Support Group (UK)
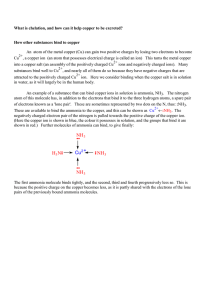
... attracted to the positively charged Cu ion. Here we consider binding when the copper salt is in solution in water, as it will largely be in the human body. An example of a substance that can bind copper ions in solution is ammonia, NH3 . The nitrogen atom of this molecule has, in addition to the ele ...
... attracted to the positively charged Cu ion. Here we consider binding when the copper salt is in solution in water, as it will largely be in the human body. An example of a substance that can bind copper ions in solution is ammonia, NH3 . The nitrogen atom of this molecule has, in addition to the ele ...
Chapter 3 Molecules, Compounds, and Chemical Equations
... 9They do not describe how many atoms, the order of attachment, or the shape. 9The formulas for ionic compounds are empirical. The empirical formula for the ionic compound fluorspar is CaCl2. This means that there is 1 Ca2+ ion for every 2 Cl− ions in the compound. The empirical formula for the molec ...
... 9They do not describe how many atoms, the order of attachment, or the shape. 9The formulas for ionic compounds are empirical. The empirical formula for the ionic compound fluorspar is CaCl2. This means that there is 1 Ca2+ ion for every 2 Cl− ions in the compound. The empirical formula for the molec ...
Fall Semester Review
... 61. In a molecule of fluorine, the two shared electrons give each fluorine atom how many electron(s) in the outer energy level? 62. The electron configuration of nitrogen is 1s2 2s2 2p 3 . How many more electrons does nitrogen need to satisfy the octet rule? 63. What group of elements satisfies the ...
... 61. In a molecule of fluorine, the two shared electrons give each fluorine atom how many electron(s) in the outer energy level? 62. The electron configuration of nitrogen is 1s2 2s2 2p 3 . How many more electrons does nitrogen need to satisfy the octet rule? 63. What group of elements satisfies the ...
The Preparation of an Explosive: Nitrogen
... iodine atoms are displacing the hydrogen atoms in ammonia, as shown. Nitrogen triiodide is highly unstable compound due to the weak bonds of the nitro gen to the iodine atoms and the size difference (nitrogen cannot hold the iodine atoms). The compound was able to be transported and handled while in ...
... iodine atoms are displacing the hydrogen atoms in ammonia, as shown. Nitrogen triiodide is highly unstable compound due to the weak bonds of the nitro gen to the iodine atoms and the size difference (nitrogen cannot hold the iodine atoms). The compound was able to be transported and handled while in ...
Atomic Structure
... ideas about atomic structure that you will have come across in an introductory chemistry course for example gcse, structure of the atom grades 6 8 - structure of the atom matter has mass and takes up space atoms are basic building blocks of matter and cannot be chemically subdivided by ordinary mean ...
... ideas about atomic structure that you will have come across in an introductory chemistry course for example gcse, structure of the atom grades 6 8 - structure of the atom matter has mass and takes up space atoms are basic building blocks of matter and cannot be chemically subdivided by ordinary mean ...
topic1
... All matter is made from about 100 different chemical elements. The Periodic Table of the Elements shows all of the known elements, arranged by increasing atomic number. Each element has a symbol. The symbol for many of the elements is one capital letter. In two-letter symbols for elements, the first ...
... All matter is made from about 100 different chemical elements. The Periodic Table of the Elements shows all of the known elements, arranged by increasing atomic number. Each element has a symbol. The symbol for many of the elements is one capital letter. In two-letter symbols for elements, the first ...
unit 8 – compound stoichiometry
... Example 8-28: A chemist heats a 300 gram sample of hydrated iron (III) nitrate until all of the water of hydration is driven off. The anhydrous (dry) compound is found to weigh 179.7 grams. What is the correct name of the hydrate? ...
... Example 8-28: A chemist heats a 300 gram sample of hydrated iron (III) nitrate until all of the water of hydration is driven off. The anhydrous (dry) compound is found to weigh 179.7 grams. What is the correct name of the hydrate? ...
Chemical reactions alter arrangements of atoms.
... A change in the state of a substance is an example of a physical change. The substance may have some different properties after a physical change, but it is still the same substance. For example, you know that water can exist in three different physical states: the solid state (ice), the liquid stat ...
... A change in the state of a substance is an example of a physical change. The substance may have some different properties after a physical change, but it is still the same substance. For example, you know that water can exist in three different physical states: the solid state (ice), the liquid stat ...
Sample Questions
... 1. The atomic mass of rhenium is 186.2. Given that 37.1% of natural rhenium is rhenium-185, what is the other stable isotope? 2. Naturally occurring element X exists in three isotopic forms: X-28 (27.977 amu, 92.23% abundance), X-29 (28.976 amu, 4.67% abundance), and X-30 (29.974 amu, 3.10% abundanc ...
... 1. The atomic mass of rhenium is 186.2. Given that 37.1% of natural rhenium is rhenium-185, what is the other stable isotope? 2. Naturally occurring element X exists in three isotopic forms: X-28 (27.977 amu, 92.23% abundance), X-29 (28.976 amu, 4.67% abundance), and X-30 (29.974 amu, 3.10% abundanc ...
Chemistry: A Molecular Approach
... oxygen for every 1.00 g of carbon carbon dioxide contains 2.67 g of oxygen for every 1.00 g of carbon since there are twice as many oxygen atoms per carbon atom in carbon dioxide than in carbon monoxide, the oxygen mass ratio should be 2 mass of oxygen that combines with 1 g of carbon in carbon diox ...
... oxygen for every 1.00 g of carbon carbon dioxide contains 2.67 g of oxygen for every 1.00 g of carbon since there are twice as many oxygen atoms per carbon atom in carbon dioxide than in carbon monoxide, the oxygen mass ratio should be 2 mass of oxygen that combines with 1 g of carbon in carbon diox ...
CHM 151LL: States of Matter: Physical and Chemical Changes
... In a pure substance every particle in the sample would look the same. In a drawing of a pure substance, every particle in the material looks the same. In a drawing of a mixture, different components of the mixture will look different. A mixture contains different atoms, molecules, and/or compounds a ...
... In a pure substance every particle in the sample would look the same. In a drawing of a pure substance, every particle in the material looks the same. In a drawing of a mixture, different components of the mixture will look different. A mixture contains different atoms, molecules, and/or compounds a ...
Chemistry: Matter and Change
... • Understand how Dalton's theory explains the conservation of mass. ...
... • Understand how Dalton's theory explains the conservation of mass. ...
Chapter 3: Stoichiometry
... One substance breaks down into two or more substances CaCO3 (s) CaO (s) + CO2 (g) 2 KClO3 (s) 2 KCl (s) + O2 (g) 2 NaN3 (s) 2 Na (s) + 3 N2 (g) ...
... One substance breaks down into two or more substances CaCO3 (s) CaO (s) + CO2 (g) 2 KClO3 (s) 2 KCl (s) + O2 (g) 2 NaN3 (s) 2 Na (s) + 3 N2 (g) ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.