6.7 Explaining the Periodic Table
... Did you notice any pattern emerging from the periodic table of Bohr–Rutherford diagrams? Mendeleev would have been fascinated to see such a startling pattern from these “family portraits” of the elements. • As you go down each family, the number of electron orbits increases—a new orbit is added wit ...
... Did you notice any pattern emerging from the periodic table of Bohr–Rutherford diagrams? Mendeleev would have been fascinated to see such a startling pattern from these “family portraits” of the elements. • As you go down each family, the number of electron orbits increases—a new orbit is added wit ...
Chemistry 11th
... Distillation under reduced pressure also involves conversion of a liquid into vapours by heating followed by condensation of the vapours thus produced by cooling under reduced pressure. In this method, the pressure acting on the system is not atmospheric pressure but is reduced with the help of a ...
... Distillation under reduced pressure also involves conversion of a liquid into vapours by heating followed by condensation of the vapours thus produced by cooling under reduced pressure. In this method, the pressure acting on the system is not atmospheric pressure but is reduced with the help of a ...
SIDE GROUP ADDITION TO THE POLYCYCLIC AROMATIC
... 300, so the PAHs were isolated from one another. The concentrations of the other molecules listed above were 5%– 25% relative to H2O, consistent with IR astronomical observations. This vapor deposition technique produces intimately mixed ices with the H2O in a high-density amorphous form that is onl ...
... 300, so the PAHs were isolated from one another. The concentrations of the other molecules listed above were 5%– 25% relative to H2O, consistent with IR astronomical observations. This vapor deposition technique produces intimately mixed ices with the H2O in a high-density amorphous form that is onl ...
Reactions and Stoichiometry Practice Problems
... 25) How many grams of NO are required to produce 145 g of N2 in the following unbalanced reaction? NH3 + ...
... 25) How many grams of NO are required to produce 145 g of N2 in the following unbalanced reaction? NH3 + ...
Follow the steps to find the number
... 3. The number of electrons in a sodium +1 ion. Multiply by the number of protons in an atom of Sn. Divide by the number of neutrons in at atom of H-2. Multiply by the number of electrons in an atom of neutral barium. 4. The number of electrons in Cu. Subtract the number of neutrons in F. Multiply by ...
... 3. The number of electrons in a sodium +1 ion. Multiply by the number of protons in an atom of Sn. Divide by the number of neutrons in at atom of H-2. Multiply by the number of electrons in an atom of neutral barium. 4. The number of electrons in Cu. Subtract the number of neutrons in F. Multiply by ...
Ministry Strand: Quantities in Chemical Reactions Teacher
... D2.1 - use appropriate terminology related to quantities in chemical reactions, including, but not limited to: stoichiometry, percentage yield, limiting reagent, mole, and atomic mass D 2.5 - calculate the corresponding mass, or quantity in moles or molecules, for any given reactant or product in a ...
... D2.1 - use appropriate terminology related to quantities in chemical reactions, including, but not limited to: stoichiometry, percentage yield, limiting reagent, mole, and atomic mass D 2.5 - calculate the corresponding mass, or quantity in moles or molecules, for any given reactant or product in a ...
General, Organic, and Biological Chemistry
... Match the type of measurement (#9-14 below) to the unit given, options A through E. A) mass B) volume C) distance D) temperature E) density ...
... Match the type of measurement (#9-14 below) to the unit given, options A through E. A) mass B) volume C) distance D) temperature E) density ...
molecular vibrations: from harmonic oscillators to pendulums
... one sees regular, circular orbits. However, for larger coupling strengths the phase space is a rich mix of regular (red, green, blue, white) and chaotic (violet) regions. ...
... one sees regular, circular orbits. However, for larger coupling strengths the phase space is a rich mix of regular (red, green, blue, white) and chaotic (violet) regions. ...
DAY
... light. By crashing protons into antiprotons or into fixed targets, researchers can create new and different particles to study. Creating a new particle, however, requires an enormous amount of energy. The Tevatron is unique because it can accelerate particles to energies higher than those of any oth ...
... light. By crashing protons into antiprotons or into fixed targets, researchers can create new and different particles to study. Creating a new particle, however, requires an enormous amount of energy. The Tevatron is unique because it can accelerate particles to energies higher than those of any oth ...
05_Lecture - HCC Learning Web
... • Percent Abundance: – Percent of atoms in a natural sample of a pure element that are a particular isotope of the element. – Distribution of isotopes in any particular sample is generally constant. – Is used to determine an atomic mass unit (amu) – 23 elements have only one naturally occurring form ...
... • Percent Abundance: – Percent of atoms in a natural sample of a pure element that are a particular isotope of the element. – Distribution of isotopes in any particular sample is generally constant. – Is used to determine an atomic mass unit (amu) – 23 elements have only one naturally occurring form ...
FINAL REVIEW Vella Name_______________ Period___
... 2. Nitrogen gas is collected over water at a temperature of 40 degrees Celsius ( v.p. of H2O = 7.38 kPa). The water levels inside and outside the collecting bottle are equal at the end of the experiment. Calculate the pressure of the nitrogen gas if the atmospheric pressure = 97.3 kPa. ...
... 2. Nitrogen gas is collected over water at a temperature of 40 degrees Celsius ( v.p. of H2O = 7.38 kPa). The water levels inside and outside the collecting bottle are equal at the end of the experiment. Calculate the pressure of the nitrogen gas if the atmospheric pressure = 97.3 kPa. ...
Unit 2.4 Understanding the Elements Listed on the Periodic Table
... After the discovery of the proton, scientists assumed that the weight of an atom was essentially that of the protons – electrons were known to contribute almost nothing to the atomic weight of the element. This approach worked until we learned how to determine the number of protons in an element. We ...
... After the discovery of the proton, scientists assumed that the weight of an atom was essentially that of the protons – electrons were known to contribute almost nothing to the atomic weight of the element. This approach worked until we learned how to determine the number of protons in an element. We ...
Structure of Atom
... Planck Quantum Theory After the failure of Electromagnetic wave theory, Max.Planck came into picture and gives few postulates as: 1. Radiant energy is emitted or absorbed not continuously but discontinuously in the form of packets called Quantum. & these quantum a re called Photons in case of light. ...
... Planck Quantum Theory After the failure of Electromagnetic wave theory, Max.Planck came into picture and gives few postulates as: 1. Radiant energy is emitted or absorbed not continuously but discontinuously in the form of packets called Quantum. & these quantum a re called Photons in case of light. ...
CHEMISTRY
... • Einstein’s theory of light’s dual nature accounted for several unexplainable phenomena but not why atomic emission spectra of elements were discontinuous rather continuous. • In 1913, Niels Bohr, a Danish physicist working in Rutherford’s laboratory, proposed a quantum model for the hydrogen atom ...
... • Einstein’s theory of light’s dual nature accounted for several unexplainable phenomena but not why atomic emission spectra of elements were discontinuous rather continuous. • In 1913, Niels Bohr, a Danish physicist working in Rutherford’s laboratory, proposed a quantum model for the hydrogen atom ...
Empirical and Molecular Formulas and Percentage Composition
... While this is true, it’s impossible to actually study this reaction at the atomic level in a chemistry laboratory; we cannot count out just one or two atoms or molecules of anything! We have to use another way to measure out the necessary quantities of chlorine and potassium iodide. The mole A mole ...
... While this is true, it’s impossible to actually study this reaction at the atomic level in a chemistry laboratory; we cannot count out just one or two atoms or molecules of anything! We have to use another way to measure out the necessary quantities of chlorine and potassium iodide. The mole A mole ...
mass number
... the nucleus. These are numbered consecutively from 1 – 7, starting from the nucleus and working outward. 2] Each energy level has a specific maximum capacity for holding electrons. 3] Energy levels can be sub-divided into sub-levels (or subshells). These are identified with letters: s, p, d, f…etc. ...
... the nucleus. These are numbered consecutively from 1 – 7, starting from the nucleus and working outward. 2] Each energy level has a specific maximum capacity for holding electrons. 3] Energy levels can be sub-divided into sub-levels (or subshells). These are identified with letters: s, p, d, f…etc. ...
Chapter 7. CHEMICAL REACTIONS
... A reaction has occurred when one or more of the following can be observed: ...
... A reaction has occurred when one or more of the following can be observed: ...
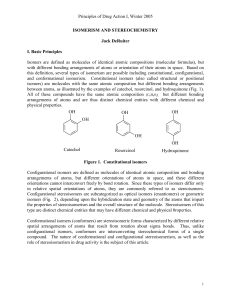
Principles of Drug Action I, Winter 2005 ISOMERISM AND
... 1813, the mineralogist Biot reported that asymmetrically cut quartz crystals rotate the plane of a beam of polarized light. It also was noted that certain organic liquids, as well as solutions of certain organic compounds, can rotate the plane of polarized light. Biot attributed this effect on plane ...
... 1813, the mineralogist Biot reported that asymmetrically cut quartz crystals rotate the plane of a beam of polarized light. It also was noted that certain organic liquids, as well as solutions of certain organic compounds, can rotate the plane of polarized light. Biot attributed this effect on plane ...
Chapter 6 Notes - Discount Flies
... Coeffecient = a whole number written in front of a substance which indicates the number of molecules that react. Tricks for balancing: 1. Write correct formulas for reactants and products first. Don’t ever change the formula of a substance once it is written correctly. 2. Balance O and H last. ...
... Coeffecient = a whole number written in front of a substance which indicates the number of molecules that react. Tricks for balancing: 1. Write correct formulas for reactants and products first. Don’t ever change the formula of a substance once it is written correctly. 2. Balance O and H last. ...
Name: ___________ Class: _____ Date: _______________ FALL
... c. sugar is dissolved in a solution. d. an unexpected color change occurs. ____ 11. Which sign does NOT indicate that a chemical change has occurred? a. color change. c. energy absorbed or released. b. dissolving in a solution. d. gas produced. ____ 12. A physical change occurs when a. milk sours. c ...
... c. sugar is dissolved in a solution. d. an unexpected color change occurs. ____ 11. Which sign does NOT indicate that a chemical change has occurred? a. color change. c. energy absorbed or released. b. dissolving in a solution. d. gas produced. ____ 12. A physical change occurs when a. milk sours. c ...
PHYSICAL SETTING CHEMISTRY
... may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 66 through 68 on the information below. In the early 1800s, John Dalton proposed an atomic theory that was based on experimental observations made by several scientists. Three concepts of Dalto ...
... may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 66 through 68 on the information below. In the early 1800s, John Dalton proposed an atomic theory that was based on experimental observations made by several scientists. Three concepts of Dalto ...
empirical formula
... ratio of atoms that always exists for that compound Example: Water – H2O Always 2 H atoms to 1 O atom ...
... ratio of atoms that always exists for that compound Example: Water – H2O Always 2 H atoms to 1 O atom ...
Sample Exercise 2.1 Illustrating the Size of an Atom
... (a) The number of protons (22) is the atomic number of the element. By referring to a periodic table or list of elements, we see that the element with atomic number 22 is titanium (Ti). The mass number of this isotope of titanium is 22 + 26 = 48 (the sum of the protons and neutrons). Because the ion ...
... (a) The number of protons (22) is the atomic number of the element. By referring to a periodic table or list of elements, we see that the element with atomic number 22 is titanium (Ti). The mass number of this isotope of titanium is 22 + 26 = 48 (the sum of the protons and neutrons). Because the ion ...
Ch02-sample-and-practice-set-2
... (a) The number of protons (22) is the atomic number of the element. By referring to a periodic table or list of elements, we see that the element with atomic number 22 is titanium (Ti). The mass number of this isotope of titanium is 22 + 26 = 48 (the sum of the protons and neutrons). Because the ion ...
... (a) The number of protons (22) is the atomic number of the element. By referring to a periodic table or list of elements, we see that the element with atomic number 22 is titanium (Ti). The mass number of this isotope of titanium is 22 + 26 = 48 (the sum of the protons and neutrons). Because the ion ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.