CST Review Part 2
... Conservation of Matter and Stoichiometry State Standard #3 The conservation of atoms in chemical reactions leads to the principle of conservation of matter and the ability to calculate the mass of products and reactants. As a basis for understanding this concept: a. Students know how to describe ch ...
... Conservation of Matter and Stoichiometry State Standard #3 The conservation of atoms in chemical reactions leads to the principle of conservation of matter and the ability to calculate the mass of products and reactants. As a basis for understanding this concept: a. Students know how to describe ch ...
Ionic vs Molecular Compounds Name Period Unit 4 – HW 1
... 16. Chemical formulas can also be written for ionic compounds. In this case, however, the formula does ...
... 16. Chemical formulas can also be written for ionic compounds. In this case, however, the formula does ...
Chapter 7 Chemical Formulas
... composed of two elements The total positive and negative charges must be equal Charges are balanced by having more than one of an ion, which is indicated by a subscript Ex. Magnesium Bromide = Mg+2 and Br -1 ~ +2 and -1 are not balanced, you need two of the bromines to balance the charge: MgBr ...
... composed of two elements The total positive and negative charges must be equal Charges are balanced by having more than one of an ion, which is indicated by a subscript Ex. Magnesium Bromide = Mg+2 and Br -1 ~ +2 and -1 are not balanced, you need two of the bromines to balance the charge: MgBr ...
Atoms and Elements: Are they Related?
... • What are the most commonly occurring elements in the food labels? • What items seemed to have the most amount of elements in them? • Can you predict what that means about the food item? • Why do you think the baby formula has such a variety of elements? • Can you predict what the other items on th ...
... • What are the most commonly occurring elements in the food labels? • What items seemed to have the most amount of elements in them? • Can you predict what that means about the food item? • Why do you think the baby formula has such a variety of elements? • Can you predict what the other items on th ...
August 2010 Regents Exam part 1
... 4 The atomic mass of titanium is 47.88 atomic mass units. This atomic mass represents the (1) total mass of all the protons and neutrons in an atom of Ti (2) total mass of all the protons, neutrons, and electrons in an atom of Ti (3) weighted average mass of the most abundant isotope of Ti (4) weigh ...
... 4 The atomic mass of titanium is 47.88 atomic mass units. This atomic mass represents the (1) total mass of all the protons and neutrons in an atom of Ti (2) total mass of all the protons, neutrons, and electrons in an atom of Ti (3) weighted average mass of the most abundant isotope of Ti (4) weigh ...
Molecules and Ions
... and type of atoms) found inside any molecule: Molecular Formula: the actual number and type of atoms in a compound, e.g. hydrogen peroxide = H2O2 Empirical Formula: the lowest whole number ratio of each type of atom in a compound e.g. hydrogen peroxide = HO ...
... and type of atoms) found inside any molecule: Molecular Formula: the actual number and type of atoms in a compound, e.g. hydrogen peroxide = H2O2 Empirical Formula: the lowest whole number ratio of each type of atom in a compound e.g. hydrogen peroxide = HO ...
Molecules and Ions
... and type of atoms) found inside any molecule: Molecular Formula: the actual number and type of atoms in a compound, e.g. hydrogen peroxide = H2O2 Empirical Formula: the lowest whole number ratio of each type of atom in a compound e.g. hydrogen peroxide = HO ...
... and type of atoms) found inside any molecule: Molecular Formula: the actual number and type of atoms in a compound, e.g. hydrogen peroxide = H2O2 Empirical Formula: the lowest whole number ratio of each type of atom in a compound e.g. hydrogen peroxide = HO ...
PCSD General Chemistry Pacing Guide
... Describe the arrangement of the modern Periodic Table, including names of important families State the general trend for and arrange elements according to: atomic size ionization energy ...
... Describe the arrangement of the modern Periodic Table, including names of important families State the general trend for and arrange elements according to: atomic size ionization energy ...
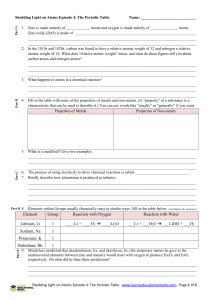
Element Group Reaction with Oxygen Reaction with Water Lithium
... 2. In the 1810s and 1820s, carbon was found to have a relative atomic weight of 12 and nitrogen a relative atomic weight of 14. What does “relative atomic weight” mean, and what do these figures tell you about carbon atoms and nitrogen atoms? _________________________________________________________ ...
... 2. In the 1810s and 1820s, carbon was found to have a relative atomic weight of 12 and nitrogen a relative atomic weight of 14. What does “relative atomic weight” mean, and what do these figures tell you about carbon atoms and nitrogen atoms? _________________________________________________________ ...
H Why - Yale University
... The values of bond dissociation energies and average bond energies, when corrected for certain “effects” (i.e. predictable errors) can lead to understanding equilibrium and rate processes through statistical mechanics. The Boltzmann factor favors minimal energy in order to provide the largest number ...
... The values of bond dissociation energies and average bond energies, when corrected for certain “effects” (i.e. predictable errors) can lead to understanding equilibrium and rate processes through statistical mechanics. The Boltzmann factor favors minimal energy in order to provide the largest number ...
Chapter 10. Chemical Bonding II. Molecular Geometry and
... Describe the bonding in CH4 molecule. experimental fact -- CH4 is tetrahedral (H-C-H angle = 109.5°) VSEPR theory "explains" this with 4 e- pairs, ∴ tetrahedral however, if only s and p orbitals are used, the angles ought to be 90° since the p orbitals are mutually perpendicular! ...
... Describe the bonding in CH4 molecule. experimental fact -- CH4 is tetrahedral (H-C-H angle = 109.5°) VSEPR theory "explains" this with 4 e- pairs, ∴ tetrahedral however, if only s and p orbitals are used, the angles ought to be 90° since the p orbitals are mutually perpendicular! ...
Exam 1 Review Sheet Honors Biology This is to be used for
... you think we completely ignore gravity on the atomic level? (Hint: why do we ignore electrons when calculating mass?) 13. The nucleus of elements larger than hydrogen obviously has more than one proton in close proximity. How can this be if the electromagnetic force is pushing these like charges ap ...
... you think we completely ignore gravity on the atomic level? (Hint: why do we ignore electrons when calculating mass?) 13. The nucleus of elements larger than hydrogen obviously has more than one proton in close proximity. How can this be if the electromagnetic force is pushing these like charges ap ...
Unit - III - E
... action, partnered to a lesser degree, etc.; thus a "co-valent bond", essentially, means that the atoms share "valence", such as is discussed in valence bond theory. In the molecule H2, the hydrogen atoms share the two electrons via covalent bonding. Covalency is greatest between atoms of similar ele ...
... action, partnered to a lesser degree, etc.; thus a "co-valent bond", essentially, means that the atoms share "valence", such as is discussed in valence bond theory. In the molecule H2, the hydrogen atoms share the two electrons via covalent bonding. Covalency is greatest between atoms of similar ele ...
Chemical Reactions - hrsbstaff.ednet.ns.ca
... What is a chemical reaction? • A chemical reaction is a chemical change where chemical substances (called reactants) react to give new chemical substances (called products). • Example – The combustion of hydrogen in oxygen is a chemical reaction which gives water. • Hydrogen and Oxygen are the reac ...
... What is a chemical reaction? • A chemical reaction is a chemical change where chemical substances (called reactants) react to give new chemical substances (called products). • Example – The combustion of hydrogen in oxygen is a chemical reaction which gives water. • Hydrogen and Oxygen are the reac ...
Chapter 3: Introduction to chemical formulas and reactivity
... formula weight. Interconvert between number of particles, moles, and mass. Understand the term percent composition and know how to calculate the percent composition of an element in a formula. Use percentage composition to determine the empirical formula of a substance. Understand how to formulate a ...
... formula weight. Interconvert between number of particles, moles, and mass. Understand the term percent composition and know how to calculate the percent composition of an element in a formula. Use percentage composition to determine the empirical formula of a substance. Understand how to formulate a ...
Chemistry 20H
... Physical reactions also include subdivision, when a substance is broken into pieces. If a rock is ground into powder, the powder is the same substance as the rock was; the subdivision has resulted only in a physical change. A chemical change results when the atoms of one or more substances are rearr ...
... Physical reactions also include subdivision, when a substance is broken into pieces. If a rock is ground into powder, the powder is the same substance as the rock was; the subdivision has resulted only in a physical change. A chemical change results when the atoms of one or more substances are rearr ...
CHEMISTRY 1
... A. Bond Energy - The average energy required for the dissociation of a bond B. Bond Length - The average distance between the two nuclei of covalently bonded atoms. C. Drawing LEWIS STRUCTURES - Electron dot structures-Review HOW TO: ...
... A. Bond Energy - The average energy required for the dissociation of a bond B. Bond Length - The average distance between the two nuclei of covalently bonded atoms. C. Drawing LEWIS STRUCTURES - Electron dot structures-Review HOW TO: ...
ert146 lect on work and power
... Another equation for working kinetics problems involving particles can be derived by integrating the equation of motion (F = ma) with respect to displacement. By substituting at = v (dv/ds) into Ft = mat, the result is integrated to yield an equation known as the principle of work and energy. This p ...
... Another equation for working kinetics problems involving particles can be derived by integrating the equation of motion (F = ma) with respect to displacement. By substituting at = v (dv/ds) into Ft = mat, the result is integrated to yield an equation known as the principle of work and energy. This p ...
Meeting no
... Entropy increases over time. Another way of stating this law is to say that heat cannot flow, on its own, from an area of cold to an area of hot. ...
... Entropy increases over time. Another way of stating this law is to say that heat cannot flow, on its own, from an area of cold to an area of hot. ...
Document
... Are attractive forces in which hydrogen that is covalently bonded to a very electronegative atom is also weakly bonded to an unshared electron pair of another ...
... Are attractive forces in which hydrogen that is covalently bonded to a very electronegative atom is also weakly bonded to an unshared electron pair of another ...
Summer Assignment
... You are among the best students at SBHS and we know that you are up to the challenge of AP Chemistry. Be forewarned that you will be working hard in this class... much harder than you have worked in other classes.... and according to past students, much harder than in any high school class you will ...
... You are among the best students at SBHS and we know that you are up to the challenge of AP Chemistry. Be forewarned that you will be working hard in this class... much harder than you have worked in other classes.... and according to past students, much harder than in any high school class you will ...