chapter 15: answers to selected problems
... both reactions break the bond between two phosphate groups. 15.67 The first reaction can be harnessed to make a molecule of ATP (from ADP and phosphate), because it produces more than 7.3 kcal of energy. 15.69 The enzymes of the electron transport chain remove electrons from NADH and FADH2, and pass ...
... both reactions break the bond between two phosphate groups. 15.67 The first reaction can be harnessed to make a molecule of ATP (from ADP and phosphate), because it produces more than 7.3 kcal of energy. 15.69 The enzymes of the electron transport chain remove electrons from NADH and FADH2, and pass ...
Origin of metabolism
... evolutionary diversification, against a background of facile and readily re-used organic chemistry. Topological modularity in the small-molecule substrate network is often associated with functional divisions in the more complex molecules that control metabolism, particularly the cofactors, showing ...
... evolutionary diversification, against a background of facile and readily re-used organic chemistry. Topological modularity in the small-molecule substrate network is often associated with functional divisions in the more complex molecules that control metabolism, particularly the cofactors, showing ...
Module 1. General principles of metabolism. Metabolism of
... D. Products of enzymatic reactions E. * Vitamins 79. Ca++ or Mg++ are most likely to be part of ___________, while Zn++ or Fe++ are present in _______________. A. * Metal-activated enzymes; metalloenzymes B. Metalloenzymes; metal-activated enzymes C. Cofactors; coenzymes D. Coenzymes; cofactors E. A ...
... D. Products of enzymatic reactions E. * Vitamins 79. Ca++ or Mg++ are most likely to be part of ___________, while Zn++ or Fe++ are present in _______________. A. * Metal-activated enzymes; metalloenzymes B. Metalloenzymes; metal-activated enzymes C. Cofactors; coenzymes D. Coenzymes; cofactors E. A ...
PDF - DigiNole! - Florida State University
... 2,5-Diketo-D-gluconic acid reductase (2,5-DKGR; E.C. 1.1.1.-) catalyzes the Nicotinamide adenine dinucleotide phosphate (NADPH)dependent stereo-specific reduction of 2,5-diketo-Dgluconate (2,5-DKG) to 2-keto-L-gulonate (2-KLG), a precursor in the industrial production of vitamin C (L-ascorbate). Mic ...
... 2,5-Diketo-D-gluconic acid reductase (2,5-DKGR; E.C. 1.1.1.-) catalyzes the Nicotinamide adenine dinucleotide phosphate (NADPH)dependent stereo-specific reduction of 2,5-diketo-Dgluconate (2,5-DKG) to 2-keto-L-gulonate (2-KLG), a precursor in the industrial production of vitamin C (L-ascorbate). Mic ...
Introduction to Carbohydrates
... metabolism of most cells, is particularly important in tissues of the nervous system. • In thiamine deficiency, the activity of these two dehydrogenase reactions is decreased, resulting in a decreased production of ATP and, thus, ...
... metabolism of most cells, is particularly important in tissues of the nervous system. • In thiamine deficiency, the activity of these two dehydrogenase reactions is decreased, resulting in a decreased production of ATP and, thus, ...
Evaluation of computational metabolic
... been identified in the genome. An enzyme E that catalyzes reaction R might not have been identified in the genome because the sequence of E has diverged so far from the sequences of other known enzymes that catalyze R that the homology cannot be detected, or because E is not evolutionarily related t ...
... been identified in the genome. An enzyme E that catalyzes reaction R might not have been identified in the genome because the sequence of E has diverged so far from the sequences of other known enzymes that catalyze R that the homology cannot be detected, or because E is not evolutionarily related t ...
Enzyme Mechanisms - Illinois Institute of Technology
... Enzyme usually works on esters too Found in eukaryotic digestive enzymes and in bacterial systems Widely-varying substrate specificities ...
... Enzyme usually works on esters too Found in eukaryotic digestive enzymes and in bacterial systems Widely-varying substrate specificities ...
Molecular Modeling of Substrate Binding in Wild
... 2,5-Diketo-D-gluconic acid reductase (2,5-DKGR; E.C. 1.1.1.-) catalyzes the Nicotinamide adenine dinucleotide phosphate (NADPH)dependent stereo-specific reduction of 2,5-diketo-Dgluconate (2,5-DKG) to 2-keto-L-gulonate (2-KLG), a precursor in the industrial production of vitamin C (L-ascorbate). Mic ...
... 2,5-Diketo-D-gluconic acid reductase (2,5-DKGR; E.C. 1.1.1.-) catalyzes the Nicotinamide adenine dinucleotide phosphate (NADPH)dependent stereo-specific reduction of 2,5-diketo-Dgluconate (2,5-DKG) to 2-keto-L-gulonate (2-KLG), a precursor in the industrial production of vitamin C (L-ascorbate). Mic ...
Enzymes:The Catalysts of Life I
... active site of lysozyme is a small groove in the enzyme surface into which the peptidoglycan fits. Lysozyme is a single polypeptide with 129 amino acid residues, but relatively few of these are directly involved in substrate binding and catalysis. Four of these are highlighted in Figure 6-2a. Substr ...
... active site of lysozyme is a small groove in the enzyme surface into which the peptidoglycan fits. Lysozyme is a single polypeptide with 129 amino acid residues, but relatively few of these are directly involved in substrate binding and catalysis. Four of these are highlighted in Figure 6-2a. Substr ...
Identification of catalytically essential amino acid residues and immobilization Rumex
... in gel, diffusion effects of the substrate and /or products [6 ]. The reduction in V m ax for immobilized enzymes as compared to the free G DH is usual. This is mainly due to their partial inactivation caused by less favorable conditions of catalysis following the immobilization process [2 3 ]. The ...
... in gel, diffusion effects of the substrate and /or products [6 ]. The reduction in V m ax for immobilized enzymes as compared to the free G DH is usual. This is mainly due to their partial inactivation caused by less favorable conditions of catalysis following the immobilization process [2 3 ]. The ...
22nd EMC Full Program - 25th Enzyme Mechanisms Conference
... Receptor tyrosine kinases (RTKs) are important cancer drug discovery targets yet the molecular underpinnings of RTK regulation, function, oncogenic activation, and drug selectivity are still not fully understood. Oncogenic receptor tyrosine kinases and clinical agents were studied to further our und ...
... Receptor tyrosine kinases (RTKs) are important cancer drug discovery targets yet the molecular underpinnings of RTK regulation, function, oncogenic activation, and drug selectivity are still not fully understood. Oncogenic receptor tyrosine kinases and clinical agents were studied to further our und ...
Raven/Johnson Biology 8e Chapter 7 – Answers 1. An autotroph is
... c. substrate-level phosphorylation d. autophosphorylation The correct answer is b— A. Answer a is incorrect. Electrons are associated with the production of ATP via the electron transport chain on the inner membrane of the mitochondria. The synthesis of ATP inside a mitochondrion is the result of ox ...
... c. substrate-level phosphorylation d. autophosphorylation The correct answer is b— A. Answer a is incorrect. Electrons are associated with the production of ATP via the electron transport chain on the inner membrane of the mitochondria. The synthesis of ATP inside a mitochondrion is the result of ox ...
Raven/Johnson Biology 8e
... c. substrate-level phosphorylation d. autophosphorylation The correct answer is b— A. Answer a is incorrect. Electrons are associated with the production of ATP via the electron transport chain on the inner membrane of the mitochondria. The synthesis of ATP inside a mitochondrion is the result of ox ...
... c. substrate-level phosphorylation d. autophosphorylation The correct answer is b— A. Answer a is incorrect. Electrons are associated with the production of ATP via the electron transport chain on the inner membrane of the mitochondria. The synthesis of ATP inside a mitochondrion is the result of ox ...
Biology, 7e (Campbell) Chapter 9: Cellular Respiration: Harvesting
... form a two-carbon compound called acetate, and 3) is bonded to coenzyme A. These three steps result in the formation of A) acetyl CoA, O2, and ATP. B) acetyl CoA, FADH2, and CO2. C) acetyl CoA, FAD, H2, and CO2. D) acetyl CoA, NADH, H+, and CO2. E) acetyl CoA, NAD+, ATP, and CO2. Topic: Concept 9.3 ...
... form a two-carbon compound called acetate, and 3) is bonded to coenzyme A. These three steps result in the formation of A) acetyl CoA, O2, and ATP. B) acetyl CoA, FADH2, and CO2. C) acetyl CoA, FAD, H2, and CO2. D) acetyl CoA, NADH, H+, and CO2. E) acetyl CoA, NAD+, ATP, and CO2. Topic: Concept 9.3 ...
Glutathione Conjugation
... !each been shown to be induced 3 -50-fold in ! !various transformed model cell lines. The ! !increased levels of GSTs may contribute to ! !multidrug resistance of tumor cells, but the causal !relationship between GST levels and drug ! !resistance is still debated. ! ...
... !each been shown to be induced 3 -50-fold in ! !various transformed model cell lines. The ! !increased levels of GSTs may contribute to ! !multidrug resistance of tumor cells, but the causal !relationship between GST levels and drug ! !resistance is still debated. ! ...
BIOSYNTHESIS OF AMINO ACIDS, NUCLEOTIDES, AND
... 4Fe-4S centers called P clusters; the remainder is present as part of a novel iron-molybdenum cofactor. A form of nitrogenase that contains vanadium rather than molybdenum has been discovered, and some bacterial species can produce both types of nitrogenase systems. The vanadium-containing enzyme ma ...
... 4Fe-4S centers called P clusters; the remainder is present as part of a novel iron-molybdenum cofactor. A form of nitrogenase that contains vanadium rather than molybdenum has been discovered, and some bacterial species can produce both types of nitrogenase systems. The vanadium-containing enzyme ma ...
The acetaminophen metabolite
... Liss et al., 2013; Pitt and Hauser, 1998) the majority were poorly nourished, had one or more chronic morbidities requiring pain relief, and often on-going sepsis. Some were alcohol abusers, had renal impairment, and/or post-operative infection and three were pregnant. The median age was 54 y (range ...
... Liss et al., 2013; Pitt and Hauser, 1998) the majority were poorly nourished, had one or more chronic morbidities requiring pain relief, and often on-going sepsis. Some were alcohol abusers, had renal impairment, and/or post-operative infection and three were pregnant. The median age was 54 y (range ...
Short-term regulation of the mammalian pyruvate dehydrogenase
... et al., 1976; Sumegi & Alkonyi, 1983). The measurements were based on the initial rate of the reaction catalyzed by PDHC. However, a far higher PDC activity was observed when the reaction rate was measured for a longer period, because in the absence of added TDP the maximum rate was achieved only a� ...
... et al., 1976; Sumegi & Alkonyi, 1983). The measurements were based on the initial rate of the reaction catalyzed by PDHC. However, a far higher PDC activity was observed when the reaction rate was measured for a longer period, because in the absence of added TDP the maximum rate was achieved only a� ...
Enzymes at work
... where A is a hydrogen acceptor molecule. Other examples of oxidoreductases are oxidases and laccases, both catalyzing the oxidation of various substrates by dioxygen, and peroxidases, catalyzing oxidations by hydrogen peroxide. Catalases are a special type, catalyzing the disproportionation reaction ...
... where A is a hydrogen acceptor molecule. Other examples of oxidoreductases are oxidases and laccases, both catalyzing the oxidation of various substrates by dioxygen, and peroxidases, catalyzing oxidations by hydrogen peroxide. Catalases are a special type, catalyzing the disproportionation reaction ...
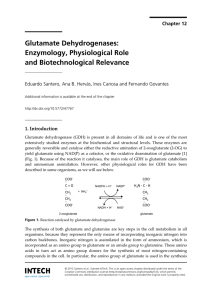
Glutamate Dehydrogenases: Enzymology, Physiological
... (Fig. 1). Because of the reaction it catalyses, the main role of GDH is glutamate catabolism and ammonium assimilation. However, other physiological roles for GDH have been described in some organisms, as we will see below. ...
... (Fig. 1). Because of the reaction it catalyses, the main role of GDH is glutamate catabolism and ammonium assimilation. However, other physiological roles for GDH have been described in some organisms, as we will see below. ...
Chapter 8: Energy generation:glycolysis
... proteins. Some enzyme cofactors are also activated carrier molecules. These include NAD+ and NADP+, each of which can carry energy in the form of a pair of electrons and a proton (H+ ion), converting the molecules into their reduced forms referred to as NADH and NADPH. The chemical equations for red ...
... proteins. Some enzyme cofactors are also activated carrier molecules. These include NAD+ and NADP+, each of which can carry energy in the form of a pair of electrons and a proton (H+ ion), converting the molecules into their reduced forms referred to as NADH and NADPH. The chemical equations for red ...
Module 2 General principles of metabolism. Мetabolism of carbohy
... E. * All reactions produce some heat. 17. Active holoenzymes are formed from ____________ in the presence of _________. A. Cofactors; proteins B. Proteins; cofactors C. * Apoenzymes; cofactors D. Apoenzymes; proteins E. Apoenzymes; inactive holoenzymes 18. An allosteric inhibitor of an enzyme usual ...
... E. * All reactions produce some heat. 17. Active holoenzymes are formed from ____________ in the presence of _________. A. Cofactors; proteins B. Proteins; cofactors C. * Apoenzymes; cofactors D. Apoenzymes; proteins E. Apoenzymes; inactive holoenzymes 18. An allosteric inhibitor of an enzyme usual ...
NH2
... ■Removal of ammonia from a.as can not be explained alone by transamination nor by oxidative deamination alone It can not be explained by transamination alone as no free ammonia is liberated nor by oxidative deamination alone as oxid. Deamination works efficiently only on glutamic acid as L- glutamat ...
... ■Removal of ammonia from a.as can not be explained alone by transamination nor by oxidative deamination alone It can not be explained by transamination alone as no free ammonia is liberated nor by oxidative deamination alone as oxid. Deamination works efficiently only on glutamic acid as L- glutamat ...
Enzyme inhibitor
... biochemistry and pharmacology. A medicinal enzyme inhibitor is often judged by its specificity (its lack of binding to other proteins) and its potency (its dissociation constant, which indicates the concentration needed to inhibit the enzyme). A high specificity and potency ensure that When an enzyme ...
... biochemistry and pharmacology. A medicinal enzyme inhibitor is often judged by its specificity (its lack of binding to other proteins) and its potency (its dissociation constant, which indicates the concentration needed to inhibit the enzyme). A high specificity and potency ensure that When an enzyme ...
Glycolysis and Gluconeogenesis
... of ATP in stage 1. Going backward in the pathway one step to glyceraldehyde 3-phosphate, we find the outcomes of the reactions catalyzed by glyceraldehyde 3-phosphate dehydrogenase and phosphoglycerate kinase to be as follows: 1. Glyceraldehyde 3-phosphate, an aldehyde, is oxidized to 3-phosphoglyce ...
... of ATP in stage 1. Going backward in the pathway one step to glyceraldehyde 3-phosphate, we find the outcomes of the reactions catalyzed by glyceraldehyde 3-phosphate dehydrogenase and phosphoglycerate kinase to be as follows: 1. Glyceraldehyde 3-phosphate, an aldehyde, is oxidized to 3-phosphoglyce ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.