2 - Physics at Oregon State University
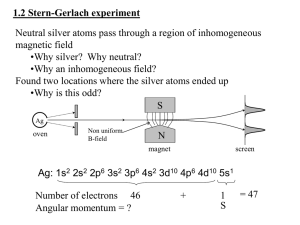
... • They thought there should be splitting with the Bohr model because they thought that the silver atom should have a h/2pi orbital angular momentum from that model, when in fact it's zero ...
... • They thought there should be splitting with the Bohr model because they thought that the silver atom should have a h/2pi orbital angular momentum from that model, when in fact it's zero ...
CHM134 General Chemistry I Semester Review – Dr. Steel This list
... This list represents the most important topics we covered in CHM134 this semester. It is not a complete list of every topic that might appear on the final exam. Formulas and Constants K = °C + 273 M D= 1 in = 2.54 cm V metric conversions ...
... This list represents the most important topics we covered in CHM134 this semester. It is not a complete list of every topic that might appear on the final exam. Formulas and Constants K = °C + 273 M D= 1 in = 2.54 cm V metric conversions ...
Georgia Physical Science Standards
... 1. Students will investigate our current understanding of the atom. a. Examine the structure of the atom in terms of: -proton, electron, and neutron locations. -atomic mass and atomic number. -atoms with different numbers of neutrons (isotopes). -explain the relationship of the proton number to the ...
... 1. Students will investigate our current understanding of the atom. a. Examine the structure of the atom in terms of: -proton, electron, and neutron locations. -atomic mass and atomic number. -atoms with different numbers of neutrons (isotopes). -explain the relationship of the proton number to the ...
L 34 Modern Physics [1]
... all the laws of physics were known – planetary motion was understood – the laws of electricity and magnetism were known – the conservation principles were established ...
... all the laws of physics were known – planetary motion was understood – the laws of electricity and magnetism were known – the conservation principles were established ...
Atomic Structure and Electron Configurations Multiple Choice PSI
... C. Angular quantum number (l) which describes the shape of an electron’s orbital D. Magnetic quantum number (ml) which describes the orbitals orientation in space 7. The Heisenberg Uncertainty Principle A. assumes that the electrons take positions predicted by Bohr's theory. B. states that the posit ...
... C. Angular quantum number (l) which describes the shape of an electron’s orbital D. Magnetic quantum number (ml) which describes the orbitals orientation in space 7. The Heisenberg Uncertainty Principle A. assumes that the electrons take positions predicted by Bohr's theory. B. states that the posit ...
Atomic Structure and Electron Configurations Multiple Choice PSI
... C. Angular quantum number (l) which describes the shape of an electron’s orbital D. Magnetic quantum number (ml) which describes the orbitals orientation in space 7. The Heisenberg Uncertainty Principle A. assumes that the electrons take positions predicted by Bohr's theory. B. states that the posit ...
... C. Angular quantum number (l) which describes the shape of an electron’s orbital D. Magnetic quantum number (ml) which describes the orbitals orientation in space 7. The Heisenberg Uncertainty Principle A. assumes that the electrons take positions predicted by Bohr's theory. B. states that the posit ...
Chapter 23 (Section 3) Pregnancy, Birth, and Childhood (Pages 735
... 3. ATOM SMALLEST unit of an ELEMENT and maintain the PROPERTIES of that element 4. MOLECULE SMALLEST unit of a COMPOUND; maintaining PROPERTIES of the compound 5. ELEMENT matter that is composed of one kind of ATOM (e.g. sulfur [S]; carbon [C]) a. each ELEMENT has its own CHARACTERISTIC chemic ...
... 3. ATOM SMALLEST unit of an ELEMENT and maintain the PROPERTIES of that element 4. MOLECULE SMALLEST unit of a COMPOUND; maintaining PROPERTIES of the compound 5. ELEMENT matter that is composed of one kind of ATOM (e.g. sulfur [S]; carbon [C]) a. each ELEMENT has its own CHARACTERISTIC chemic ...
Student choices of models of the atom - OSU Physics
... nearly 1000 undergraduates at the Ohio State University who were taking one of the OSU physics courses. The survey is an attempt to determine student ideas about the nature of reality. We used the concept of the photon as a common thread to probe students’ understanding of a wide range of topics in ...
... nearly 1000 undergraduates at the Ohio State University who were taking one of the OSU physics courses. The survey is an attempt to determine student ideas about the nature of reality. We used the concept of the photon as a common thread to probe students’ understanding of a wide range of topics in ...
Notes-15 - KSU Physics
... For the present purpose, each of these orbitals can be viewed like a hydrogenic orbital. In a more advanced model, like the so-called Hatree-Fock model, the concept of the orbitals is retained, but the potential each electron sees is not a Coulomb potential, but rather a central field potential whic ...
... For the present purpose, each of these orbitals can be viewed like a hydrogenic orbital. In a more advanced model, like the so-called Hatree-Fock model, the concept of the orbitals is retained, but the potential each electron sees is not a Coulomb potential, but rather a central field potential whic ...
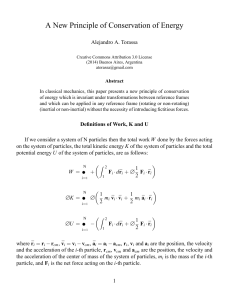
A New Principle of Conservation of Energy
... In a system of N particles, the total work W done by the forces acting on the system of particles is equal to the change in the total kinetic energy K of the system of particles. W = +∆ K In a system of N particles, the total work W done by the conservative forces acting on the system of particles i ...
... In a system of N particles, the total work W done by the forces acting on the system of particles is equal to the change in the total kinetic energy K of the system of particles. W = +∆ K In a system of N particles, the total work W done by the conservative forces acting on the system of particles i ...
The History of Quantum Mechanics
... • The exclusion principle (No two electrons may exist in the same quantum state) provided the reason for electrons in atoms being arranged in shells with the maximum number of electrons being 2, 8, 18, 32… etc, from the first to the nth shell. This principle is significant for the fact that it exp ...
... • The exclusion principle (No two electrons may exist in the same quantum state) provided the reason for electrons in atoms being arranged in shells with the maximum number of electrons being 2, 8, 18, 32… etc, from the first to the nth shell. This principle is significant for the fact that it exp ...
Determination of the Atomic Weight of Magnesium CHEM 101
... the balance. Other potential sources of experimental uncertainty are: the reaction might not be complete; if not enough time was allowed for total oxidation, less than complete oxidation of the magnesium might have, in part, reacted with nitrogen in the air (incorrect reaction); the magnesium oxide ...
... the balance. Other potential sources of experimental uncertainty are: the reaction might not be complete; if not enough time was allowed for total oxidation, less than complete oxidation of the magnesium might have, in part, reacted with nitrogen in the air (incorrect reaction); the magnesium oxide ...
Atomic Structure MC Review_ corrected
... C. Angular quantum number (l) which describes the shape of an electron’s orbital D. Magnetic quantum number (ml) which describes the orbitals orientation in space 7. The Heisenberg Uncertainty Principle A. assumes that the electrons take positions predicted by Bohr's theory. B. states that the posit ...
... C. Angular quantum number (l) which describes the shape of an electron’s orbital D. Magnetic quantum number (ml) which describes the orbitals orientation in space 7. The Heisenberg Uncertainty Principle A. assumes that the electrons take positions predicted by Bohr's theory. B. states that the posit ...
eastern illinois university
... c. VCl2, CrCl2 d. VCl2, TaCl2 e. VCl2 and FeCl3 19. Mercury(II) thiocyanate Hg(SCN)2 was once used to make the white color in fireworks. The %S by mass in this compound is: a. 6.2% b. 10.1% c. 12.4% d. 20.2% e. 24.8% 20. A compound composed of the elements carbon and hydrogen is 82.66% carbon and 17 ...
... c. VCl2, CrCl2 d. VCl2, TaCl2 e. VCl2 and FeCl3 19. Mercury(II) thiocyanate Hg(SCN)2 was once used to make the white color in fireworks. The %S by mass in this compound is: a. 6.2% b. 10.1% c. 12.4% d. 20.2% e. 24.8% 20. A compound composed of the elements carbon and hydrogen is 82.66% carbon and 17 ...
Physics 535 lectures notes: 1 * Sep 4th 2007
... 5V approach the barrier. What percentage of electrons will tunnel through the barrier? 3) Consider a infinite 3D box potential with L2=2L1 and L3=4L1. What are the quantum numbers of the lowest degenerate energy levels? List, ordered by energy, the quantum numbers and energies of all the levels up t ...
... 5V approach the barrier. What percentage of electrons will tunnel through the barrier? 3) Consider a infinite 3D box potential with L2=2L1 and L3=4L1. What are the quantum numbers of the lowest degenerate energy levels? List, ordered by energy, the quantum numbers and energies of all the levels up t ...
Key equations exercises
... differ from one another? In what respects are they the same? 2.33 (a) What isotope is used as the standard in establishing the atomic mass scale? (b) The atomic weight of boron is reported as 10.81, yet no atom of boron has the mass of 10.81 amu. Explain. 2.34 (a) What is the mass in amu of a carb ...
... differ from one another? In what respects are they the same? 2.33 (a) What isotope is used as the standard in establishing the atomic mass scale? (b) The atomic weight of boron is reported as 10.81, yet no atom of boron has the mass of 10.81 amu. Explain. 2.34 (a) What is the mass in amu of a carb ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.


![L 33 Modern Physics [1] Modern Physics](http://s1.studyres.com/store/data/003217156_1-265c5a519e2bca3f33717b4abd842898-300x300.png)
![L 34 Modern Physics [1]](http://s1.studyres.com/store/data/008622077_1-047a8df5b8f51427a7d951942e25e95f-300x300.png)