star test review
... 29) Which properties are most common in nonmetals? (a) low ionization energy and low electronegativity (b) low ionization energy and high electronegativity (c) high ionization energy and low electronegativity (d) high ionization energy and high electronegativity ...
... 29) Which properties are most common in nonmetals? (a) low ionization energy and low electronegativity (b) low ionization energy and high electronegativity (c) high ionization energy and low electronegativity (d) high ionization energy and high electronegativity ...
High School Chemistry
... Science language students should use: Atom, element, nucleus, proton, neutron, electron, metalloid, periodic table, isotope, metal, half-life, fission, fusion, nonmetal, quanta, photon, wavelength, radioactivity, spectrum Science Benchmark Atoms form bonds with other atoms by transferring or sharing ...
... Science language students should use: Atom, element, nucleus, proton, neutron, electron, metalloid, periodic table, isotope, metal, half-life, fission, fusion, nonmetal, quanta, photon, wavelength, radioactivity, spectrum Science Benchmark Atoms form bonds with other atoms by transferring or sharing ...
Unit 8 Waves: Quantum Mechanical Waves
... An electron moving in a circular orbit requires acceleration. This should cause light to be radiated, so that energy would be released and the electron would spiral in to the nucleus. (Of course, this is not observed to happen; undisturbed atoms can last “forever.”) As in Planck’s case for energy of ...
... An electron moving in a circular orbit requires acceleration. This should cause light to be radiated, so that energy would be released and the electron would spiral in to the nucleus. (Of course, this is not observed to happen; undisturbed atoms can last “forever.”) As in Planck’s case for energy of ...
Document
... "Once at the end of a colloquium I heard Debye saying something like: Schrödinger, you are not working right now on very important problems anyway. Why don't you tell us sometime about that thesis of de Broglie? "So in one of the next colloquia, Schrödinger gave a beautifully clear account of how de ...
... "Once at the end of a colloquium I heard Debye saying something like: Schrödinger, you are not working right now on very important problems anyway. Why don't you tell us sometime about that thesis of de Broglie? "So in one of the next colloquia, Schrödinger gave a beautifully clear account of how de ...
Atomic Physics
... accounts for the interaction between the nucleus and the electron. We then discovered that the family of stationary states, which are the solutions of the Schrödinger equation can be completely characterized by three quantum numbers n, , and m . However, we also found that the state of the electron ...
... accounts for the interaction between the nucleus and the electron. We then discovered that the family of stationary states, which are the solutions of the Schrödinger equation can be completely characterized by three quantum numbers n, , and m . However, we also found that the state of the electron ...
Chapter 7. Atomic Physics
... accounts for the interaction between the nucleus and the electron. We then discovered that the family of stationary states, which are the solutions of the Schrödinger equation can be completely characterized by three quantum numbers n, , and m . However, we also found that the state of the electron ...
... accounts for the interaction between the nucleus and the electron. We then discovered that the family of stationary states, which are the solutions of the Schrödinger equation can be completely characterized by three quantum numbers n, , and m . However, we also found that the state of the electron ...
Homework Assignment for CHEM 5591 Professor JM Weber
... Aluminum (you don’t need a microstate table for this problem). Which term describes the ground state of Al? ...
... Aluminum (you don’t need a microstate table for this problem). Which term describes the ground state of Al? ...
Physical Science - Edgemead High School
... effect produced in the ear due to the sound of a particular frequency. Pitch is directly proportional to frequency. Relate the loudness of a sound to both the amplitude of a sound wave and the sensitivity of the human ear. Loudness is a subjective term describing the strength of the ear's percepti ...
... effect produced in the ear due to the sound of a particular frequency. Pitch is directly proportional to frequency. Relate the loudness of a sound to both the amplitude of a sound wave and the sensitivity of the human ear. Loudness is a subjective term describing the strength of the ear's percepti ...
A Brief History of Modern Physics and the development of the
... consists of a small, heavy, positively-charged nucleus, surrounded by small € But there is a problem with the classical theory of this light electrons. nuclear atom: An electron in orbit about a nucleus is accelerating and, according to Maxwell's equations, an accelerating charge must radiate (give ...
... consists of a small, heavy, positively-charged nucleus, surrounded by small € But there is a problem with the classical theory of this light electrons. nuclear atom: An electron in orbit about a nucleus is accelerating and, according to Maxwell's equations, an accelerating charge must radiate (give ...
From quantum to quantum computer
... Academy of Science gave him financial support to set up an Physics Institute. The fund was actually donated by Carlsberg Brewery (beer)! The Institute quickly became the center of quantum science in the 1920s and 1930s, due to Bohr’s genius and his personality. ...
... Academy of Science gave him financial support to set up an Physics Institute. The fund was actually donated by Carlsberg Brewery (beer)! The Institute quickly became the center of quantum science in the 1920s and 1930s, due to Bohr’s genius and his personality. ...
15.3 - Department of Physics
... and objects to be considered as external. To use field concept instead of Coulomb’s law we split the Universe into two parts: • the charges that are the sources of the field • the charge that is affected by that field ...
... and objects to be considered as external. To use field concept instead of Coulomb’s law we split the Universe into two parts: • the charges that are the sources of the field • the charge that is affected by that field ...
Scientific Method - Virtual Medical Academy
... * Shiny, ductile. * Good conductors of heat and electricity. Nonmetals *Located to the right of the heavy line. * Dull and brittle. * Poor conductors. ...
... * Shiny, ductile. * Good conductors of heat and electricity. Nonmetals *Located to the right of the heavy line. * Dull and brittle. * Poor conductors. ...
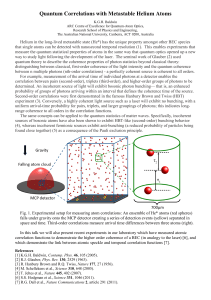
Quantum Correlations with Metastable Helium Atoms
... experiment (3). Conversely, a highly coherent light source such as a laser will exhibit no bunching, with a uniform arrival-time probability for pairs, triplets, and larger groupings of photons; this indicates longrange coherence to all orders in the correlation functions. The same concepts can be a ...
... experiment (3). Conversely, a highly coherent light source such as a laser will exhibit no bunching, with a uniform arrival-time probability for pairs, triplets, and larger groupings of photons; this indicates longrange coherence to all orders in the correlation functions. The same concepts can be a ...
lecture_CH1-2review_chem121pikul
... General, Organic, & Biological Chemistry Janice Gorzynski Smith ...
... General, Organic, & Biological Chemistry Janice Gorzynski Smith ...
Chapter 27: Summary
... behavior of such particles is very different from the behavior of everyday objects. Among other things, these tiny objects exhibit both a wave nature and a particle nature. Black body radiation Black body radiation is the radiation, in the form of electromagnetic waves, which emanates from a warm ob ...
... behavior of such particles is very different from the behavior of everyday objects. Among other things, these tiny objects exhibit both a wave nature and a particle nature. Black body radiation Black body radiation is the radiation, in the form of electromagnetic waves, which emanates from a warm ob ...
Common Chemical Formula List
... Balancing chemical equations isn't difficult, once you know the way to do it. Start by finding out how many atoms of each type are on each side of the equation. Some teachers recommend making a little table listing the numbers of each atom for the left hand side and for the right hand side. Next, lo ...
... Balancing chemical equations isn't difficult, once you know the way to do it. Start by finding out how many atoms of each type are on each side of the equation. Some teachers recommend making a little table listing the numbers of each atom for the left hand side and for the right hand side. Next, lo ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI
... State any two postulates of quantum mechanics. Prove that the square of the angular momentum commutes with its components. What is meant by degeneracy of an energy level? What is an orthonormal basis? With an example explain simultaneous eigenfunctions. If A and B are two operators, then show that [ ...
... State any two postulates of quantum mechanics. Prove that the square of the angular momentum commutes with its components. What is meant by degeneracy of an energy level? What is an orthonormal basis? With an example explain simultaneous eigenfunctions. If A and B are two operators, then show that [ ...
Arrangement of Electrons in Atoms
... occupied by one e- before pairing up e-. All single occupied orbitals must have same ...
... occupied by one e- before pairing up e-. All single occupied orbitals must have same ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.