Views on Atomic Stru..
... Wave mechanics provides a probability of where an electron will be in certain regions of an atom The Born interpretation: The square of a wave function (y2) gives the probability of finding an electron in a small volume of space around the atom (orbital) ...
... Wave mechanics provides a probability of where an electron will be in certain regions of an atom The Born interpretation: The square of a wave function (y2) gives the probability of finding an electron in a small volume of space around the atom (orbital) ...
Quantum Physics
... Planck's quantum theory. Explain why the classical curve is incorrect. What was Planck's key assumption in his quantum model? (What did he quantize?) CLICK FOR ANSWER ...
... Planck's quantum theory. Explain why the classical curve is incorrect. What was Planck's key assumption in his quantum model? (What did he quantize?) CLICK FOR ANSWER ...
Chapter 2
... a. Car Engine - gasoline used to run motor to move car Chemical Energy (gas) ---> motion (20%) + heat (79%) + sound (1%) b. Human Body - food used to move body, digest, think, etc. Chemical Energy (food/glucose) ---> physiology (80%) + heat (20%) ...
... a. Car Engine - gasoline used to run motor to move car Chemical Energy (gas) ---> motion (20%) + heat (79%) + sound (1%) b. Human Body - food used to move body, digest, think, etc. Chemical Energy (food/glucose) ---> physiology (80%) + heat (20%) ...
Hydrogen Atom Simulator – Exercises
... energy levels, but these are very close together and those lines not shown on the simulator. o The Lε line has an energy of -13.22 eV and is in range two. The ionization energy for an electron in the ground state is 13.6 eV and so that is the correct range 3 boundary. ...
... energy levels, but these are very close together and those lines not shown on the simulator. o The Lε line has an energy of -13.22 eV and is in range two. The ionization energy for an electron in the ground state is 13.6 eV and so that is the correct range 3 boundary. ...
Atomic and Molecular Physics for Physicists Ben-Gurion University of the Negev
... J in the lowest energy state of the EXCITED atom (this will be the one interacting with the light as the light is red-detuned) ...
... J in the lowest energy state of the EXCITED atom (this will be the one interacting with the light as the light is red-detuned) ...
CHEMISTRY 102B Name Hour Exam II March 19, 2015 Signature
... Which of the following best evaluates the statement “The 1st ionization energy for an oxygen atom is lower than the 1st ionization energy for a nitrogen atom”? a) It is consistent with the general trend relating changes in ionization energy across a period from left to right because it is easier to ...
... Which of the following best evaluates the statement “The 1st ionization energy for an oxygen atom is lower than the 1st ionization energy for a nitrogen atom”? a) It is consistent with the general trend relating changes in ionization energy across a period from left to right because it is easier to ...
Boltzmann/Saha Equation Problems/Questions
... state. So, we are asking how many different states j are there with the same energy Ej ? In this problem, we are comparing the only possible ionization states of Hydrogen: neutral and singly ionized (HII). Since a hydrogen ion is just a proton, there is only one possible energy state so gr+1 = 1. Ne ...
... state. So, we are asking how many different states j are there with the same energy Ej ? In this problem, we are comparing the only possible ionization states of Hydrogen: neutral and singly ionized (HII). Since a hydrogen ion is just a proton, there is only one possible energy state so gr+1 = 1. Ne ...
File
... a. demonstrate an understanding of the terms atom, element, ion, molecule, compound, empirical and molecular formulae b. write balanced equations (full and ionic) for simple reactions, including the use of state symbols c. demonstrate an understanding of the terms relative atomic mass, amount of sub ...
... a. demonstrate an understanding of the terms atom, element, ion, molecule, compound, empirical and molecular formulae b. write balanced equations (full and ionic) for simple reactions, including the use of state symbols c. demonstrate an understanding of the terms relative atomic mass, amount of sub ...
Budiansky Cover
... "Once at the end of a colloquium I heard Debye saying something like: Schrödinger, you are not working right now on very important problems anyway. Why don't you tell us sometime about that thesis of de Broglie? "So in one of the next colloquia, Schrödinger gave a beautifully clear account of how de ...
... "Once at the end of a colloquium I heard Debye saying something like: Schrödinger, you are not working right now on very important problems anyway. Why don't you tell us sometime about that thesis of de Broglie? "So in one of the next colloquia, Schrödinger gave a beautifully clear account of how de ...
Chapter 2 - Las Positas College
... so the atom is first boosted to an excited state (one of the orbital electrons jumps to a higher state) and then it can emit a photon as it drops to a lower state. If the excited electron drops all the way back to its lowest state (leaving the atom in the ground state) then the energy of the photon ...
... so the atom is first boosted to an excited state (one of the orbital electrons jumps to a higher state) and then it can emit a photon as it drops to a lower state. If the excited electron drops all the way back to its lowest state (leaving the atom in the ground state) then the energy of the photon ...
Chapter 12 - "Chemical Formulas and Equations"
... – The mole is derived from the following information. • Atomic weights are an average of the relative masses of all of the isotopes of the given element. • The number of C-12 atoms in exactly 12.00 g of C12 is 6.02 X 1023. – This called Avogadro’s number. ...
... – The mole is derived from the following information. • Atomic weights are an average of the relative masses of all of the isotopes of the given element. • The number of C-12 atoms in exactly 12.00 g of C12 is 6.02 X 1023. – This called Avogadro’s number. ...
40.4: Angular Momenta and Magnetic Dipole
... absorption of photons. To produce laser light, one must have more photons emitted than absorbed; that is, one must have a situation in which stimulated emission dominates. Thus, one needs more atoms in the excited state than in the ground state, as in Fig. 40-19b. ...
... absorption of photons. To produce laser light, one must have more photons emitted than absorbed; that is, one must have a situation in which stimulated emission dominates. Thus, one needs more atoms in the excited state than in the ground state, as in Fig. 40-19b. ...
E - Purdue Physics
... Suppose that these are the quantized energy levels (K+U) for an atom. Initially the atom is in its ground state (symbolized by a dot). An electron with kinetic energy 6 eV collides with the atom and excites it. What is the remaining kinetic energy of the electron? ...
... Suppose that these are the quantized energy levels (K+U) for an atom. Initially the atom is in its ground state (symbolized by a dot). An electron with kinetic energy 6 eV collides with the atom and excites it. What is the remaining kinetic energy of the electron? ...
wlq10
... • measurement changes observed system so that parameter measured is subsequently definite • conjugate parameters cannot be simultaneously definite • process measure A, measure B not the same as measure B, measure A ...
... • measurement changes observed system so that parameter measured is subsequently definite • conjugate parameters cannot be simultaneously definite • process measure A, measure B not the same as measure B, measure A ...
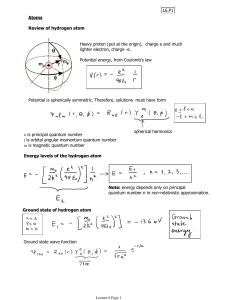
Lecture 6 - physics.udel.edu
... Potential is spherically symmetric. Therefore, solutions must have form ...
... Potential is spherically symmetric. Therefore, solutions must have form ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.