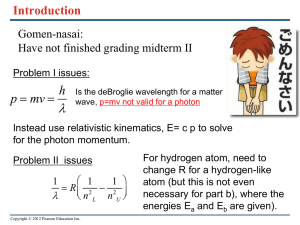
Catalyst Activity (in your notebook)
... mass – total mass of reactants = total mass of products ...
... mass – total mass of reactants = total mass of products ...
1 - High School Teachers
... 2. electric fields 3. electric current 4. electric circuits 5. magnetism 6. magnetic field property 7. electromagnetic induction 8. electromagnetic waves 9. the nature of light 10. reflection and refraction of light 11. interference and diffraction 12. discovery of the atomic nucleus 13. quantum the ...
... 2. electric fields 3. electric current 4. electric circuits 5. magnetism 6. magnetic field property 7. electromagnetic induction 8. electromagnetic waves 9. the nature of light 10. reflection and refraction of light 11. interference and diffraction 12. discovery of the atomic nucleus 13. quantum the ...
Bonding Challenge
... Station 2 (Get in “shape”) 1) (a) Draw the Lewis electron-dot structures for CO32-, CO2, and CO, including resonance structures where appropriate. (b) Put the three species in order of increasing C-O bond length? Explain the reason for your answer. (c) Predict the molecular shapes for the three spe ...
... Station 2 (Get in “shape”) 1) (a) Draw the Lewis electron-dot structures for CO32-, CO2, and CO, including resonance structures where appropriate. (b) Put the three species in order of increasing C-O bond length? Explain the reason for your answer. (c) Predict the molecular shapes for the three spe ...
toolkit - The Open University
... device (such as a cathode ray tube), the anode is given a positive charge by external means; it therefore attracts negatively-charged electrons from the partially-evacuated chamber. Compare with cathode. antineutrino The antiparticle of a neutrino. antiparticle Elementary particles and their antipar ...
... device (such as a cathode ray tube), the anode is given a positive charge by external means; it therefore attracts negatively-charged electrons from the partially-evacuated chamber. Compare with cathode. antineutrino The antiparticle of a neutrino. antiparticle Elementary particles and their antipar ...
quantum1
... dx dx conjugate (a ib ) (a ib ) if z a ib, then : z * z (a ib )( a ib ) a i b ...
... dx dx conjugate (a ib ) (a ib ) if z a ib, then : z * z (a ib )( a ib ) a i b ...
Chem Regents 2015 A Few Things
... Temperature reprents (NOT: is equal to) the average molecular kinetic energy. Heat is the amount of total molecular kinetic energy. Objects with the same temperature but different mass or heat capacity have different amounts of total energy. ...
... Temperature reprents (NOT: is equal to) the average molecular kinetic energy. Heat is the amount of total molecular kinetic energy. Objects with the same temperature but different mass or heat capacity have different amounts of total energy. ...
www.theallpapers.com
... touching each other. The dynamic equilibria between solid-liquid and liquid-gas. Vapour pressure as the result of molecules colliding with the sides of the vessel. The alternating oppositely charged ions in 3 dimensions in ionic solids allows a strong attraction between them. The continuous, 3dimens ...
... touching each other. The dynamic equilibria between solid-liquid and liquid-gas. Vapour pressure as the result of molecules colliding with the sides of the vessel. The alternating oppositely charged ions in 3 dimensions in ionic solids allows a strong attraction between them. The continuous, 3dimens ...
Atomic Theory (2
... 12.) What is the difference between an excited state and ground state? 13.) Describe the limitations of the Bohr’s model of the atom and how quantum model provides a more accurate picture of electron arrangement. . ...
... 12.) What is the difference between an excited state and ground state? 13.) Describe the limitations of the Bohr’s model of the atom and how quantum model provides a more accurate picture of electron arrangement. . ...
PHYS-2020: General Physics II Course Lecture Notes Section IX Dr. Donald G. Luttermoser
... showed that light (or any E/M radiation) can self-propagate, it took another 50 years before Einstein demonstrated this with the Special Theory of Relativity in 1905. Prior to this, light was assumed to propagate on a medium in space called the Ether. f ) In the late-1800s, Michelson and Morley trie ...
... showed that light (or any E/M radiation) can self-propagate, it took another 50 years before Einstein demonstrated this with the Special Theory of Relativity in 1905. Prior to this, light was assumed to propagate on a medium in space called the Ether. f ) In the late-1800s, Michelson and Morley trie ...
Atomic structure BV
... The black body radiation "Blackbody radiation" or "cavity radiation" refers to an object or system which absorbs all radiation incident upon it and re-radiates energy which is characteristic of this radiating system only, not dependent upon the type of radiation which is incident upon it. ...
... The black body radiation "Blackbody radiation" or "cavity radiation" refers to an object or system which absorbs all radiation incident upon it and re-radiates energy which is characteristic of this radiating system only, not dependent upon the type of radiation which is incident upon it. ...
3-D Schrodinger`s Equation, Particle inside a 3
... To study how the x-ray spectra of atoms indicate the structure of these atoms ...
... To study how the x-ray spectra of atoms indicate the structure of these atoms ...
IUPAC Periodic Table Quantum Mechanics Consistent
... hypothetical process in which an atom is progressively “built up” from its predecessor, by adding one proton and one or more neutrons to the nucleus plus one electron to the outermost free atomic orbital [5]. The Aufbau is similar to building a house, following the blueprint. The periodic table is b ...
... hypothetical process in which an atom is progressively “built up” from its predecessor, by adding one proton and one or more neutrons to the nucleus plus one electron to the outermost free atomic orbital [5]. The Aufbau is similar to building a house, following the blueprint. The periodic table is b ...
PowerPoint 演示文稿
... Because the radioactive half-life of a given radioisotope is not affected by temperature, physical or chemical state, or any other influence of the environment outside the nucleus, then radioactive samples continue to decay at a predictable rate. If determinations or reasonable estimates of the orig ...
... Because the radioactive half-life of a given radioisotope is not affected by temperature, physical or chemical state, or any other influence of the environment outside the nucleus, then radioactive samples continue to decay at a predictable rate. If determinations or reasonable estimates of the orig ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.