* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Unit Five: Periodic Table Families
Survey
Document related concepts
Transcript
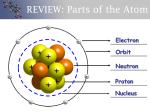
Unit Five: Periodic Table Families Unit Goals: Examine trends in behavior over period families Understand how the periodic table is structured Warm-up Questions: “Why do trends occur in the periodic table?” Assigned Readings: Glencoe pages 172 – 194, 904 – 945 http://en.wikibooks.org/wiki/General_Chemistry/Periodicity_and_Electron_Configurations http://en.wikibooks.org/wiki/General_Chemistry/Chemistries_of_Various_Elements/Group_1 http://en.wikibooks.org/wiki/General_Chemistry/Chemistries_of_Various_Elements/Group_2 http://en.wikibooks.org/wiki/General_Chemistry/Chemistries_of_Various_Elements/Group_13 http://en.wikibooks.org/wiki/General_Chemistry/Chemistries_of_Various_Elements/Group_14 http://en.wikibooks.org/wiki/General_Chemistry/Chemistries_of_Various_Elements/Group_15 http://en.wikibooks.org/wiki/General_Chemistry/Chemistries_of_Various_Elements/Group_16 http://en.wikibooks.org/wiki/General_Chemistry/Chemistries_of_Various_Elements/Group_17 http://en.wikibooks.org/wiki/General_Chemistry/Chemistries_of_Various_Elements/Group_18 http://en.wikibooks.org/wiki/General_Chemistry/Chemistries_of_Various_Elements/Hydrogen http://en.wikibooks.org/wiki/General_Chemistry/Chemistries_of_Various_Elements/Transition_ Metals Problem Set One: Assigned Problems: 1. Read all wikibook and textbook sections 2. Write a 2 – 3 paragraph summary of your assigned periodic family. How are the elements in the family similar? a. Ammine: Alkali metals b. Sarah: Alkaline earth metals c. Basil: Boron family d. Amirah: Carbon family e. Farhia: Nitrogen family f. Dina: Oxygen family g. Mohammed N: Halogens h. Aisha: Noble gases i. Mariam: Hydrogen j. Aniqa: Coinage metals k. Mohamed T: Zinc family l. Nima: Platinum family m. Fatima: Iron, Cobalt, and Nickel 3. Write three questions related to your periodic family Problem Set Two: Assigned Problems: 1. 2. 3. 4. 5. 6. How many valence electrons does Magnesium have? How many does Calcium have? How many levels (like two in 2s) does Potassium have? How many does Gallium have? Which block (s, p, d, or f) is group 2 in? What does that mean? How many families are in the p-block? How many families are in the d-block? Why? How many elements are in the d-block? What element in group one has the largest atomic radius? Which halogen has the smallest atomic radius? Which one of those is bigger? Why? 7. Which element has the larger ionic radius of these pairs: Why? a. Li vs. Be b. N vs. O c. K vs. Na 8. Use table 6.5 to answer this question. List the ionization energy required to remove one through five electrons in Carbon. Why does it jump so much at the fifth one? 9. What atom has the most electronegativity? The least electronegativity? 10. Name and explain four reasons for periodic trends. Which one is your favorite? Lecture Summary: Periodic Law o When arranged in order by atomic mass, elements have repeating chemical and physical properties Periodic table structure o Columns are called families or groups 18 families Groups 3 to 12 are called the transition metals o Rows are called periods 7 periods o Metals Elements towards the left of the table o Nonmetals Elements towards the upper right side of the table o Metalloids Some metallic properties, some non-metal properties Between the region of metals and non-metals o Organized by electron configuration Every element in a family has the same number of valence electrons All elements in a period have the same quantum number (same level of shells) s-Block elements Groups 1 and 2 + Helium p-Block Groups on right of the table d-Block Transition metals f-Block Inner transition metals Reasons for trends o Electron shielding Electrons block pull from nucleus o Electron to electron repulsion Causes electrons to spread apart o Coulomb’s Law Distance causes decrease in forces on order n2 o Effective nuclear charge The nucleus becomes more positively charged with more protons Periodic trends o Atomic radius Decreases left to right Because of increased positive charge in the nucleus Increases top to bottom Because of additional energy levels added As levels are added, close electrons “shield” the charge pull of further electrons o Ionic radius The more electrons an atom loses, the smaller it gets Groups one, two, thirteen and sometimes fourteen get smaller as they ionize The more electrons an atom gains, the larger it gets Groups in the p-block get larger to the left as they ionize Ionic radius increases as you go down the table o Ionization energy Energy required to remove an electron from an atom First ionization refers to removing the first atom, second refers to removing the second atom, etc Increases for each successive atoms; jumps when removing in levels lower than the valence shell Decreases top to bottom Electrons are further away from the nucleus Increases left to right o Electronegativity Ability to attract electrons Decreases from top to bottom Increases from left to right Groups o Hydrogen At STP has lowest density of all gases One valence electron Shares properties of Alkali metals and Halogens Three isotopes o Alkali Metals Group 1 Silvery, metallic Soft Very low densities o o o o Low melting points Highly reactive; not found in nature in free form One valence electron Form +1 ions Atomic radii and ionic radii increase downwards Electronegativity decreases downwards Alkaline Earth Metals Group 2 Silvery-white, metallic Soft, low density Density increases down table Low melting points Two valence electrons Forms +2 ions Smaller atomic and ionic radii than alkali metals Higher electronegativity than alkali metals Transition Metals Groups 3 to twelve Good conductors of heat and electricity Ductile, malleable High densities High melting points All solid at room temperature except Mercury Many paramagnetic: Attracted to magnetic fields Some ferromagnetic: Can make their own magnetic field More unpaired d electrons = harder, higher melting point, higher density Little variation to electronegativity and atomic size due to shielding Can lose both s and d electrons to form +1+/2+3 and higher ions Boron group Silvery white, except Boron which is black All metals, except Boron is a metalloid All are lightweight and soft except Boron which is extremely hard All solid at room temperatures 3 valence electrons, starts filling the p orbital All except Boron lose three electrons to form ions Some can lose only one to form ions Boron only participates in covalent bonding Atomic and ion radii increase down the group Ionization energy decreases down the group Carbon Group Metallic character increases down the group Carbon nonmetal Silicon and Germanium are metalloids Tin and lead are metals Moving down the group melting points decrease Large variety of forms and structures available o o o o Allotropes Four valence electrons Largely covalent bonds; Can give up or gain four Atomic and ionic radii increase down the group Similar ionization energy and electronegativity throughout group; except Carbon Many real world applications Nitrogen Group Increase in metallic properties as they go down Nitrogen and Phosphorous are nonmetals Arsenic and Antimony are metalloids Bismuth is a metal Boiling point and density increase down the table Also has large variety of possible physical combinations Five valence electrons -3, +3, +5 common ion formations Ionization energy and electronegative decreases down as you go down the group Atomic and ionic radii increase as you go down the table Oxygen Group All solids at room temperature except oxygen which is a gas Common allotropic forms Oxygen, sulfur, and selenium are nonmetals Tellurium and polonium are metals Melting points increase down group, except polonium Density increases down the group Six valence electrons Many different ions formed Electronegativity and ionization energy increase going down the group Halogens Fluorine and chlorine are gases at room temperature Bromine is liquid Iodine is solid Melting points increase going down the group Seven valence electrons Noble Gases Colorless, odorless gases Nonmetals Very low melting points; increase down the group Eight valence electrons except Helium; stable octet Helium has two valence electrons. Full shell, still very stable Exist as single atoms Highest first ionization; not reactive