Evolution of the Atomic Concept and the Beginnings of Modern
... In fact, although the forces binding atoms together in molecules cannot be properly understood without quantum mechanics, many of these forces are "short range" electrical forces - forces between bodies having overall electrical neutrality, but distorted charge distributions. These forces could defi ...
... In fact, although the forces binding atoms together in molecules cannot be properly understood without quantum mechanics, many of these forces are "short range" electrical forces - forces between bodies having overall electrical neutrality, but distorted charge distributions. These forces could defi ...
Emission Line Spectra and the Rydberg Constant
... appears yellow because of intense emission lines in the region of the sodium spectrum. The hydrogen spectrum is of particular theoretical interest because hydrogen, having only one proton and one electron, is the simplest of all atoms. Niels Bohr (1885 – 1962) developed a theory for the hydrogen ato ...
... appears yellow because of intense emission lines in the region of the sodium spectrum. The hydrogen spectrum is of particular theoretical interest because hydrogen, having only one proton and one electron, is the simplest of all atoms. Niels Bohr (1885 – 1962) developed a theory for the hydrogen ato ...
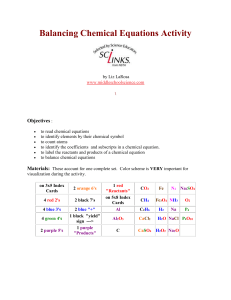
Balancing Chemical Equations Activity by Liz LaRosa www
... The index cards are a bit time consuming to create. I had some students help at lunch time for a few days. Once done, you can laminate them and have them forever! The materials account for one complete set which is good for 2-3 students to use. Print activity cards on card stock instead of making in ...
... The index cards are a bit time consuming to create. I had some students help at lunch time for a few days. Once done, you can laminate them and have them forever! The materials account for one complete set which is good for 2-3 students to use. Print activity cards on card stock instead of making in ...
Wk-11-14
... Atomic Mass Unit. Pay attention—this is where Avogadro's number comes from. •Earlier we said "Let one atom of H have 1 atomic mass unit" •Now, we have a problem, because H has 3 isotopes: •So.....we cannot use "hydrogen" as it usually exists (mixed isotopes) for our mass standard. •We must purify i ...
... Atomic Mass Unit. Pay attention—this is where Avogadro's number comes from. •Earlier we said "Let one atom of H have 1 atomic mass unit" •Now, we have a problem, because H has 3 isotopes: •So.....we cannot use "hydrogen" as it usually exists (mixed isotopes) for our mass standard. •We must purify i ...
Structure of Molecules and Compounds | Principles of Biology from
... atoms that share one pair of electrons. Consider the element carbon. It has four valence electrons. Carbon requires four additional electrons to reach a stable configuration. It can gain these electrons, for example, by combining with four hydrogen atoms. Each hydrogen atom has one electron in its o ...
... atoms that share one pair of electrons. Consider the element carbon. It has four valence electrons. Carbon requires four additional electrons to reach a stable configuration. It can gain these electrons, for example, by combining with four hydrogen atoms. Each hydrogen atom has one electron in its o ...
Electrostatics Review What is the charge of one electron?
... neutral wall, which demonstrates charge…. ...
... neutral wall, which demonstrates charge…. ...
1_Quantum theory_ introduction and principles
... The sun has a number of holes in its corona from which high energy particles (e-, p+, n0) stream out with enormous velocity. These particles are thrown out through our solar system, and the phenomenon is called solar wind. A part of this solar wind meets the earth’s magneto sphere, the solar wind pa ...
... The sun has a number of holes in its corona from which high energy particles (e-, p+, n0) stream out with enormous velocity. These particles are thrown out through our solar system, and the phenomenon is called solar wind. A part of this solar wind meets the earth’s magneto sphere, the solar wind pa ...
Astrophysics by Jonathan Chan
... explain that cathode ray tubes allowed the manipulation of a stream of charged particles The Cathode Ray Tube: Highly evacuated glass tube (to reduce obstruction/collisions) containing two electrodes High voltage applied across the electrodes Cathode rays (streams of electrons) flow from the c ...
... explain that cathode ray tubes allowed the manipulation of a stream of charged particles The Cathode Ray Tube: Highly evacuated glass tube (to reduce obstruction/collisions) containing two electrodes High voltage applied across the electrodes Cathode rays (streams of electrons) flow from the c ...
File
... a. Examine the structure of the atom in terms of i) proton, electron, and system for naming types of matter. neutron locations. ii) atomic mass and atomic number. iii) atoms with b. Predict formulas for stable binary ionic compounds based on balance of different numbers of neutrons (isotopes). iv) e ...
... a. Examine the structure of the atom in terms of i) proton, electron, and system for naming types of matter. neutron locations. ii) atomic mass and atomic number. iii) atoms with b. Predict formulas for stable binary ionic compounds based on balance of different numbers of neutrons (isotopes). iv) e ...
quant13
... • To fully understand these, need a relativistic theory of the electron – The Dirac equation, chapter 16 • For hydrogen-like atoms, we will solve this exactly • For other atoms, relativistic corrections must be approximated • Since states 2s/2p are not degenerate for these atoms, corrections not imp ...
... • To fully understand these, need a relativistic theory of the electron – The Dirac equation, chapter 16 • For hydrogen-like atoms, we will solve this exactly • For other atoms, relativistic corrections must be approximated • Since states 2s/2p are not degenerate for these atoms, corrections not imp ...
Zagazig University
... (2) Since the insulating material used has a dielectric strength is larger than that of air, so the maximum potential difference of the capacitor is increased. (3) The capacitance of a capacitor with given dimensions is increased by inserting the dielectric sheet between its plates (C) A copper wire ...
... (2) Since the insulating material used has a dielectric strength is larger than that of air, so the maximum potential difference of the capacitor is increased. (3) The capacitance of a capacitor with given dimensions is increased by inserting the dielectric sheet between its plates (C) A copper wire ...
Self-Consistent Supercell Band-Structure Calculations for the
... has its maximum for the energy value of the inter section of N v Px(E) and N v p (E) in Figure 3. Beyond this energy value Qv p (E) and Qv p (E) approach each other. For Cd the intersection point £ c, for which Qv pJ E c) = Qv Pz(Ec), is beyond the energy range displayed in Figure 4. F or the impur ...
... has its maximum for the energy value of the inter section of N v Px(E) and N v p (E) in Figure 3. Beyond this energy value Qv p (E) and Qv p (E) approach each other. For Cd the intersection point £ c, for which Qv pJ E c) = Qv Pz(Ec), is beyond the energy range displayed in Figure 4. F or the impur ...
Identical Particles ( + problems 34
... expression for just one of them? It is a consequence of the so-called scale invariance of the problem. In the case of one oscillator of the frequency ω0 , there is a characteristic scale of energy, h̄ω0 , so that the answers can be written as some non-trivial functions of dimensionless variable T /h ...
... expression for just one of them? It is a consequence of the so-called scale invariance of the problem. In the case of one oscillator of the frequency ω0 , there is a characteristic scale of energy, h̄ω0 , so that the answers can be written as some non-trivial functions of dimensionless variable T /h ...
Assignment-21 Conduction Phenomenon is
... Any missing data may be suitably assumed and stated. n= ...
... Any missing data may be suitably assumed and stated. n= ...
Spinning Electrons and the Structure of Spectra
... same angular momentum J but different azimuthal quantum numbers K. Consequently, the orbits will penetrate to different distances from the nucleus, so that the screening of the nuclear charge by the other electrons in the atom will have different effects. This screening effect will, however, be the ...
... same angular momentum J but different azimuthal quantum numbers K. Consequently, the orbits will penetrate to different distances from the nucleus, so that the screening of the nuclear charge by the other electrons in the atom will have different effects. This screening effect will, however, be the ...
Chapter 9 – Many Electron Atoms
... In practice one uses a basis set. The accuracy of a HF calculation is limited by the size of the basis set. Hartree-‐Fock results can be qualitatively wrong when you expect degeneracies between gr ...
... In practice one uses a basis set. The accuracy of a HF calculation is limited by the size of the basis set. Hartree-‐Fock results can be qualitatively wrong when you expect degeneracies between gr ...
Unit 10 – The Mole
... A mole is a counting number. One mole is a specific number of atoms, molecules, or formula units. A mole is ALWAYS ____________________________. This number was discovered by a scientist named ____________________________. So we chemists call this number Avogadro’s Number. ...
... A mole is a counting number. One mole is a specific number of atoms, molecules, or formula units. A mole is ALWAYS ____________________________. This number was discovered by a scientist named ____________________________. So we chemists call this number Avogadro’s Number. ...
File - Mrs. Hille`s FunZone
... Energy is quantized. It comes in chunks. Quanta - the amount of energy needed to move from one energy level to another. Since the energy of an atom is never “in between” there must be a quantum leap in energy. ...
... Energy is quantized. It comes in chunks. Quanta - the amount of energy needed to move from one energy level to another. Since the energy of an atom is never “in between” there must be a quantum leap in energy. ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.