Chapter 16 Notes
... •Protons have positive charge, neutrons are neutral •Mass of proton (and neutron) 1800 x mass of electron • Electrons have negative charge and are attracted to nucleus • Charge of electron is equal in magnitude to that of proton • Normal atom is neutral • Ion is atom that has gained or lost one or ...
... •Protons have positive charge, neutrons are neutral •Mass of proton (and neutron) 1800 x mass of electron • Electrons have negative charge and are attracted to nucleus • Charge of electron is equal in magnitude to that of proton • Normal atom is neutral • Ion is atom that has gained or lost one or ...
Intrinsic Semiconductors
... In this course you have learnt the following At very low temperatures, semiconductors are like insulators as there are no free carriers in their conduction band. As temperature is raised, thermal excitation of carriers takes place to the conduction band leading to non-zero conductivity. Such semico ...
... In this course you have learnt the following At very low temperatures, semiconductors are like insulators as there are no free carriers in their conduction band. As temperature is raised, thermal excitation of carriers takes place to the conduction band leading to non-zero conductivity. Such semico ...
Laser Physics I
... Light and Electromagnetic Waves Light is one form of electromagnetic radiation. Electromagnetic radiation, which transports energy from point to point at the velocity of light, can be described in terms of both wave and particle "pictures" or "models." This is the famous "wave-particle" duality ...
... Light and Electromagnetic Waves Light is one form of electromagnetic radiation. Electromagnetic radiation, which transports energy from point to point at the velocity of light, can be described in terms of both wave and particle "pictures" or "models." This is the famous "wave-particle" duality ...
Chapter 6
... energy is derived by replacing M with the total mass of the solid. This recoil energy is practically zero and the gamma ray can be absorbed by another atomic nucleus of the same element in the solid. This phenomenon is called the Mössbauer effect. When a radiation source or an absorber is moving, th ...
... energy is derived by replacing M with the total mass of the solid. This recoil energy is practically zero and the gamma ray can be absorbed by another atomic nucleus of the same element in the solid. This phenomenon is called the Mössbauer effect. When a radiation source or an absorber is moving, th ...
Chapter 4 powerpoint presentation
... The Schrödinger Wave Equation • In 1926, Austrian physicist Erwin Schrödinger developed an equation that treated electrons in atoms as waves. • Together with the Heisenberg uncertainty principle, the Schrödinger wave equation laid the foundation for modern quantum theory. • Quantum theory describes ...
... The Schrödinger Wave Equation • In 1926, Austrian physicist Erwin Schrödinger developed an equation that treated electrons in atoms as waves. • Together with the Heisenberg uncertainty principle, the Schrödinger wave equation laid the foundation for modern quantum theory. • Quantum theory describes ...
Name:
... Unit 9 Study Guide (U9SG)- you must write on your own paper (There are 28 Questions) Remember to study your goals and terms for each section. Look over your worksheets and in class practice problems. If you need additional conversion practice, there are extra practice problems on the in class practi ...
... Unit 9 Study Guide (U9SG)- you must write on your own paper (There are 28 Questions) Remember to study your goals and terms for each section. Look over your worksheets and in class practice problems. If you need additional conversion practice, there are extra practice problems on the in class practi ...
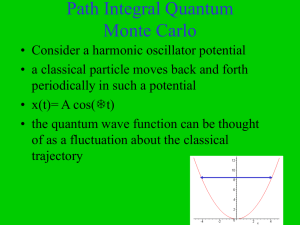
Path Integral Quantum Monte Carlo
... • This expression is similar to a partition function Z in statistical mechanics • the probability factor e- E in statistical mechanics is the analogue of e- E in quantum mechanics • =N plays the role of inverse temperature =1/kT ...
... • This expression is similar to a partition function Z in statistical mechanics • the probability factor e- E in statistical mechanics is the analogue of e- E in quantum mechanics • =N plays the role of inverse temperature =1/kT ...
DCE Sample Paper 6 - Entrance
... 42. A light spring of force constant 8 Nm-1 is cut into two equal halves and the two are connected in parallel; the equivalent force constant of the system is A. 16 Nm-1 B. 32 Nm-1 C. 8 Nm-1 D. 24 Nm- 1 43. A light spring of constant k is cut into two equal parts. The spring constant of each part is ...
... 42. A light spring of force constant 8 Nm-1 is cut into two equal halves and the two are connected in parallel; the equivalent force constant of the system is A. 16 Nm-1 B. 32 Nm-1 C. 8 Nm-1 D. 24 Nm- 1 43. A light spring of constant k is cut into two equal parts. The spring constant of each part is ...
Ц(Ш) Ш = .ЦЦ + Ц . Ъ(Ш) Ш
... The normal force N is a force perpendicular to the plane of contact with a rigid object. The frictional force is the force parallel to the plane of contact. We distinguish the case of static friction when there is no slipping and kinetic friction when there is relative motion ...
... The normal force N is a force perpendicular to the plane of contact with a rigid object. The frictional force is the force parallel to the plane of contact. We distinguish the case of static friction when there is no slipping and kinetic friction when there is relative motion ...
paper 1 - ResearchGate
... energies of a particle in a two dimensional square box as a function of the quantum numbers nX, nY, the electron mass m, and the dimensions of the box a? b) Assume for the purpose of this calculation that the square box has sides a=1nm. Assume also that at most two electrons can occupy a single ener ...
... energies of a particle in a two dimensional square box as a function of the quantum numbers nX, nY, the electron mass m, and the dimensions of the box a? b) Assume for the purpose of this calculation that the square box has sides a=1nm. Assume also that at most two electrons can occupy a single ener ...
Organic Chemistry
... Organic Chemistry: What is it? • 1780: Organic compounds are very complex and only obtained from living sources (vitalism 生机说) • Vitalism: Belief that a "magic" vital force, present in plants and animals, is necessary for the synthesis of organic compounds • 1789: Antoine Laurent Lavoisier observed ...
... Organic Chemistry: What is it? • 1780: Organic compounds are very complex and only obtained from living sources (vitalism 生机说) • Vitalism: Belief that a "magic" vital force, present in plants and animals, is necessary for the synthesis of organic compounds • 1789: Antoine Laurent Lavoisier observed ...
2 - IS MU
... It is just like the ground state orbital for the isotropic oscillator, but with a rescaled size. This is reminescent of the well-known scaling for the ground state of the helium atom. Next, the total energy is calculated for this orbital ...
... It is just like the ground state orbital for the isotropic oscillator, but with a rescaled size. This is reminescent of the well-known scaling for the ground state of the helium atom. Next, the total energy is calculated for this orbital ...
Lecture Notes3 - Haldia Institute of Technology
... the potential difference was increased further, it increased and the spur became maximum in the set for 54V at ...
... the potential difference was increased further, it increased and the spur became maximum in the set for 54V at ...
Monday, Feb. 14, 2005
... • Pauli proposed an additional particle emitted in bdecays – No one saw this particle in experiment • Difficult to detect ...
... • Pauli proposed an additional particle emitted in bdecays – No one saw this particle in experiment • Difficult to detect ...
High School Knowledge Exam – Study Guide
... Chemical Change examples: Reactions between chemicals, burning (fire reacts with something), color change (caused by reaction b/w chemicals) Dalton’s Atomic Theory 1) All matter is made up of very small, discrete particles called atoms 2) All atoms of a given element are identical, and the atoms of ...
... Chemical Change examples: Reactions between chemicals, burning (fire reacts with something), color change (caused by reaction b/w chemicals) Dalton’s Atomic Theory 1) All matter is made up of very small, discrete particles called atoms 2) All atoms of a given element are identical, and the atoms of ...
1 The Mole 6.02 X 10 23
... • Molecular Mass/Molecular Weight: If you have a single molecule, mass is measured in amu’s instead of grams. But, the molecular mass/weight is the same numerical value as 1 mole of molecules. Only the units are different. (This is the beauty of Avogadro’s Number!) ...
... • Molecular Mass/Molecular Weight: If you have a single molecule, mass is measured in amu’s instead of grams. But, the molecular mass/weight is the same numerical value as 1 mole of molecules. Only the units are different. (This is the beauty of Avogadro’s Number!) ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.