Quantum Phases and Topological States in Optical Lattices
... x⃗j )d3 x and the interaction energy U = g |w(⃗x)|4 d3 x [2, 3, 4]. As seen in the expression, the Hamiltonian consists of two crucial terms. The first term in the hamiltonian is known as the hopping term which describes the tunneling of atoms between neighboring sites. The strength is characterized ...
... x⃗j )d3 x and the interaction energy U = g |w(⃗x)|4 d3 x [2, 3, 4]. As seen in the expression, the Hamiltonian consists of two crucial terms. The first term in the hamiltonian is known as the hopping term which describes the tunneling of atoms between neighboring sites. The strength is characterized ...
Chapter 3
... Classical physics failed to completely explain the phenomenon. Assumed that radiant energy was continuous; that is, could be emitted or absorbed in any amount. Max Planck suggested that radiant energy is only emitted or absorbed in discrete quantities, like small packages or bundles. A quantum of ...
... Classical physics failed to completely explain the phenomenon. Assumed that radiant energy was continuous; that is, could be emitted or absorbed in any amount. Max Planck suggested that radiant energy is only emitted or absorbed in discrete quantities, like small packages or bundles. A quantum of ...
eOVERm Lab manual PDF.
... is an incandescent source of electrons. Near the source of electrons are electrodes (circular capacitor plates) for setting up a voltage potential. This accelerates the electrons into a beam of known potential difference V and energy eV per electron. The last electrode that the electrons in the beam ...
... is an incandescent source of electrons. Near the source of electrons are electrodes (circular capacitor plates) for setting up a voltage potential. This accelerates the electrons into a beam of known potential difference V and energy eV per electron. The last electrode that the electrons in the beam ...
1s + 2p
... A time-dependent wavefunction looks just like the spatial s we have been talking about, except that it is multiplied by eit = cos(t) + i sin(t), where i = (-1), is the energy (in frequency units) of the spatial wavefunction and t is time. In many cases this makes no difference, because whe ...
... A time-dependent wavefunction looks just like the spatial s we have been talking about, except that it is multiplied by eit = cos(t) + i sin(t), where i = (-1), is the energy (in frequency units) of the spatial wavefunction and t is time. In many cases this makes no difference, because whe ...
Helium atom in metallic electron gases: A comparative study
... In order to discuss briefly the atomic collision of slow He and Al or Mg lattice atoms in free-electron-like (kF ≃ 0.9 and kF ≃ 0.7, respectively) metals [5] at small impact parameters, we apply quantum mechanic adiabatic perturbation theory for charge-exchange processes. We note, parenthetically, t ...
... In order to discuss briefly the atomic collision of slow He and Al or Mg lattice atoms in free-electron-like (kF ≃ 0.9 and kF ≃ 0.7, respectively) metals [5] at small impact parameters, we apply quantum mechanic adiabatic perturbation theory for charge-exchange processes. We note, parenthetically, t ...
Final exam 2007
... Name (last name first): _____________________________________________ I.D. Number last 4: _________________________________________________ Note: There are 18 questions in this exam. Fill in your answer in the blank space provided immediately following each question. 1/2 point will be subtracted eve ...
... Name (last name first): _____________________________________________ I.D. Number last 4: _________________________________________________ Note: There are 18 questions in this exam. Fill in your answer in the blank space provided immediately following each question. 1/2 point will be subtracted eve ...
Fundamentals
... In the case of an elemental material, such as copper, a particle is a single atom. Because the mass of a single atom is very small, atomic mass is usually specified in atomic mass units (amu). One amu is equal to 1.6605402 X 10-24 g (1 g/ 6.022137 X 1023),or about the mass of a single hydrogen atom. ...
... In the case of an elemental material, such as copper, a particle is a single atom. Because the mass of a single atom is very small, atomic mass is usually specified in atomic mass units (amu). One amu is equal to 1.6605402 X 10-24 g (1 g/ 6.022137 X 1023),or about the mass of a single hydrogen atom. ...
Spectrum of quasistable states in a strong infrared
... The situation depicted in Fig. 3(a) is obviously different from Figs. 3(b)–3(d). The first excited state holds nearly all electrons that survive the IR field. This actually corresponds to the abnormal peak in Fig. 2(a). The launch energy is close to the first excited energy level and the IR intensit ...
... The situation depicted in Fig. 3(a) is obviously different from Figs. 3(b)–3(d). The first excited state holds nearly all electrons that survive the IR field. This actually corresponds to the abnormal peak in Fig. 2(a). The launch energy is close to the first excited energy level and the IR intensit ...
Chemical Stoichiometry
... 3. Divide each value of moles by the smallest of the values. 4. Multiply each number by an integer to obtain all whole numbers. Copyright©2000 by Houghton Mifflin Company. All rights reserved. ...
... 3. Divide each value of moles by the smallest of the values. 4. Multiply each number by an integer to obtain all whole numbers. Copyright©2000 by Houghton Mifflin Company. All rights reserved. ...
Chapter 1 - Manual Science Chemistry/Physics
... Overview: Defines matter and contrasts major physical and chemical changes that matter can undergo. This section also outlines the basic form of a chemical equation and describes how matter is classified. Objectives: Distinguish between the physical properties and chemical properties of matter ...
... Overview: Defines matter and contrasts major physical and chemical changes that matter can undergo. This section also outlines the basic form of a chemical equation and describes how matter is classified. Objectives: Distinguish between the physical properties and chemical properties of matter ...
A persistent particle ontology for QFT in terms of the Dirac sea
... Forthcoming in the British Journal for the Philosophy of Science We show that the Bohmian approach in terms of persisting particles that move on continuous trajectories following a deterministic law can be literally applied to QFT. By means of the Dirac sea model – exemplified in the electron sector ...
... Forthcoming in the British Journal for the Philosophy of Science We show that the Bohmian approach in terms of persisting particles that move on continuous trajectories following a deterministic law can be literally applied to QFT. By means of the Dirac sea model – exemplified in the electron sector ...
03 nanoparticles part 7 File - e-learning
... • by deposition of a solid from a condensing vapor stream • by deposition of a product after chemical reaction in vapor phase. The techniques require the vaporization of the material: not commonly used for ceramics, due to their low vapor pressure values. ...
... • by deposition of a solid from a condensing vapor stream • by deposition of a product after chemical reaction in vapor phase. The techniques require the vaporization of the material: not commonly used for ceramics, due to their low vapor pressure values. ...
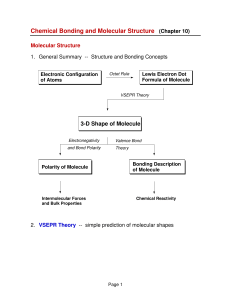
3-D Shape of Molecule
... *accounts for paramagnetism of O2 (VB theory fails here!) 4. Delocalized Molecular Orbitals By combining AO's from three or more atoms, it is possible to generate MO's that are "delocalized" over three or more atoms ...
... *accounts for paramagnetism of O2 (VB theory fails here!) 4. Delocalized Molecular Orbitals By combining AO's from three or more atoms, it is possible to generate MO's that are "delocalized" over three or more atoms ...
8.5DF: Chemical Formulas and Equations
... reacting with water to produce carbon dioxide gas. This gas produces the “holes” in the cake that give the cake its light, fluffy texture. A similar type of reaction occurs when baking soda is mixed with vinegar. Work with your child to investigate, either online or via textbook, the chemical formul ...
... reacting with water to produce carbon dioxide gas. This gas produces the “holes” in the cake that give the cake its light, fluffy texture. A similar type of reaction occurs when baking soda is mixed with vinegar. Work with your child to investigate, either online or via textbook, the chemical formul ...
Name: Northwest Vista College Chem 1311
... the stopcock is opened, given that the atmospheric pressure is 755 mmHg? Note that the gas in the round bulb contains a gas that supports the given height, h, of mercury ...
... the stopcock is opened, given that the atmospheric pressure is 755 mmHg? Note that the gas in the round bulb contains a gas that supports the given height, h, of mercury ...
Class 23
... • Exam 2 is next week (Thu., 7:30-9:00pm) Topics: TZD, Chapters 3-5, 11.9, 11.10 • Bring your own formula sheet. • No written HW due for next week but reading assignments are due as usual. ...
... • Exam 2 is next week (Thu., 7:30-9:00pm) Topics: TZD, Chapters 3-5, 11.9, 11.10 • Bring your own formula sheet. • No written HW due for next week but reading assignments are due as usual. ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.