CP Chemistry Final Review – Chap. 10-19
... Identify the four symbols used to specify physical states for substances in a reaction. Know how to use coefficients to properly balance an equation. Understand the five classifications of chemical reactions. Use the activity series to predict which single replacement reactions are possible. ...
... Identify the four symbols used to specify physical states for substances in a reaction. Know how to use coefficients to properly balance an equation. Understand the five classifications of chemical reactions. Use the activity series to predict which single replacement reactions are possible. ...
Quantum Effects in White Dwarfs - The Dartmouth Undergraduate
... less than 1.4 times the mass of our sun. In general, a white dwarf is comprised of two components: a gas of dense electrons and the positively charged nuclei, which are the product of nuclear fusion. The internal structure of a white dwarf, and indeed its very existence, depends upon the quantum nat ...
... less than 1.4 times the mass of our sun. In general, a white dwarf is comprised of two components: a gas of dense electrons and the positively charged nuclei, which are the product of nuclear fusion. The internal structure of a white dwarf, and indeed its very existence, depends upon the quantum nat ...
Chapter 1 Chemistry: Matter and Measurement
... Example: Solve the following problems and state the answers with the proper number of significant figures. a) Calculate the area of an object with a length of 1.345 m and a width of 0.057 m. ...
... Example: Solve the following problems and state the answers with the proper number of significant figures. a) Calculate the area of an object with a length of 1.345 m and a width of 0.057 m. ...
Electron Configuration of Atoms
... • The principal energy level number, the number that comes before the sublevel letter designation, is the same as the period number for the s and p sublevels. • For the d sublevels, the principal energy level number is one less than the period number. Why? ...
... • The principal energy level number, the number that comes before the sublevel letter designation, is the same as the period number for the s and p sublevels. • For the d sublevels, the principal energy level number is one less than the period number. Why? ...
matter and - cloudfront.net
... Matter with a uniform and definite composition (also called a pure substance). All samples of a substance have identical physical properties. ...
... Matter with a uniform and definite composition (also called a pure substance). All samples of a substance have identical physical properties. ...
Chapter 19 part 1
... • Electrical energy can be used to force a non-spontaneous reaction to occur (e.g. electrolysis) ...
... • Electrical energy can be used to force a non-spontaneous reaction to occur (e.g. electrolysis) ...
October 17
... A neutron of mass mOverview n and speed vni undergoes a head-on elastic collision with a carbon nucleus of mass initially atrest ...
... A neutron of mass mOverview n and speed vni undergoes a head-on elastic collision with a carbon nucleus of mass initially atrest ...
Chemistry Log Books - Social Circle City Schools
... 1. Students will receive an AKS review sheet for the upcoming unit (usually after the last unit test). This sheet should then be glued/taped to fit the page in the composition log book. Students will read the AKS listed at the top of the page to see what they will be learning in the upcoming unit. 2 ...
... 1. Students will receive an AKS review sheet for the upcoming unit (usually after the last unit test). This sheet should then be glued/taped to fit the page in the composition log book. Students will read the AKS listed at the top of the page to see what they will be learning in the upcoming unit. 2 ...
the Planck mass is incredibly larger than
... we have been able to use to create a single particle. Thus, in addition to the fact that the elementary particles we know have masses with no obvious relation to each other, if they have any particular relation to the Planck mass, it is for now simply some incredibly small fractional number to which ...
... we have been able to use to create a single particle. Thus, in addition to the fact that the elementary particles we know have masses with no obvious relation to each other, if they have any particular relation to the Planck mass, it is for now simply some incredibly small fractional number to which ...
Solutions - faculty.ucmerced.edu
... force of gravity on each atom is balanced by an outward pressure force due to the heat of the nuclear reactions in core. But after all the hydrogen “fuel” is consumed by nuclear fusion, the pressure force drops and the star undergoes gravitational collapse until it becomes a neutron star. In a neutr ...
... force of gravity on each atom is balanced by an outward pressure force due to the heat of the nuclear reactions in core. But after all the hydrogen “fuel” is consumed by nuclear fusion, the pressure force drops and the star undergoes gravitational collapse until it becomes a neutron star. In a neutr ...
Aalborg Universitet Beyond the Modern Physics and Cosmological Equations
... While the classical, wavelike behavior of light interference and diffraction has been easily observed in undergraduate laboratories for many years, explicit observation of the quantum nature of light i.e., photons is much more difficult. For example, while well-known phenomena such as the photoelect ...
... While the classical, wavelike behavior of light interference and diffraction has been easily observed in undergraduate laboratories for many years, explicit observation of the quantum nature of light i.e., photons is much more difficult. For example, while well-known phenomena such as the photoelect ...
Unit 15 Static Electricity
... become charged by friction. • The paint particles contain like charges thus they spread out when they are sprayed on a car body. • The charged paint particles will be attracted to the metallic car body. ...
... become charged by friction. • The paint particles contain like charges thus they spread out when they are sprayed on a car body. • The charged paint particles will be attracted to the metallic car body. ...
MackMadison
... sections for dark matter with nucleons Model-independent constraint, based on Earth’s heat flow ...
... sections for dark matter with nucleons Model-independent constraint, based on Earth’s heat flow ...
ELECTRON THEORY AND MAGNETISM
... compounds or mixtures. When two or more atoms combine, they form a molecule. If these atoms are not all alike, then the substance formed is a compound. Oxygen and hydrogen are both examples of an element. When one oxygen atom and two hydrogen atoms unite, they form a molecule of water. Water is a co ...
... compounds or mixtures. When two or more atoms combine, they form a molecule. If these atoms are not all alike, then the substance formed is a compound. Oxygen and hydrogen are both examples of an element. When one oxygen atom and two hydrogen atoms unite, they form a molecule of water. Water is a co ...
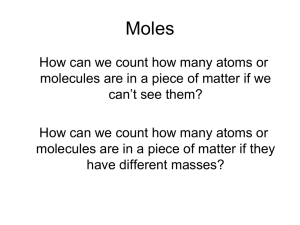
Moles - tamchemistryhart
... Example of # moles = M x L How many moles of CaCl2 are in 250. mL of 2.0 M CaCl2? How many grams is this? Begin with the value you are given in the problem (250. mL), then use molarity of CaCl2 (2.0 M) as a conversion factor. ...
... Example of # moles = M x L How many moles of CaCl2 are in 250. mL of 2.0 M CaCl2? How many grams is this? Begin with the value you are given in the problem (250. mL), then use molarity of CaCl2 (2.0 M) as a conversion factor. ...
Solve
... does not have the nitrogen-to-oxygen ratio correct and it would imply that only one molecule, composed of two nitrogen and three oxygen atoms, was shown in the figure, not the seven molecules that are actually depicted. Finally, (d) a mixture of N2 and O3 cannot be correct because there are no pure ...
... does not have the nitrogen-to-oxygen ratio correct and it would imply that only one molecule, composed of two nitrogen and three oxygen atoms, was shown in the figure, not the seven molecules that are actually depicted. Finally, (d) a mixture of N2 and O3 cannot be correct because there are no pure ...
A molecular orbital method for inorganic molecules: application to
... nonpolarizable core (perfect screening). The consequence of invoking this latter approximation has been foundz4to be a need to correct for penetration effects which modify Z*, eq 18, in the diagonal elements. Diagonal Hamiltonian matrix elements computed with neglect of inner orbitals cannot and sho ...
... nonpolarizable core (perfect screening). The consequence of invoking this latter approximation has been foundz4to be a need to correct for penetration effects which modify Z*, eq 18, in the diagonal elements. Diagonal Hamiltonian matrix elements computed with neglect of inner orbitals cannot and sho ...
Help us improve Wikipedia by supporting it financially
... leaving the ash. In other words, in combustion the fatty earth burns away. In 1661, Robert Boyle showed that there were more than just four classical elements as the ancients had assumed.[8] The first modern list of chemical elements was given in Antoine Lavoisier's 1789 Elements of Chemistry, which ...
... leaving the ash. In other words, in combustion the fatty earth burns away. In 1661, Robert Boyle showed that there were more than just four classical elements as the ancients had assumed.[8] The first modern list of chemical elements was given in Antoine Lavoisier's 1789 Elements of Chemistry, which ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.