Experiments
... As a charged particle traverses a medium it excites the atoms (or molecules) in the the medium. In certain materials called scintillators a small fraction energy released when the atoms or molecules de-excite goes into light. ENERGY IN LIGHT OUT The use of materials that scintillate is one of the ...
... As a charged particle traverses a medium it excites the atoms (or molecules) in the the medium. In certain materials called scintillators a small fraction energy released when the atoms or molecules de-excite goes into light. ENERGY IN LIGHT OUT The use of materials that scintillate is one of the ...
1210.0414v1
... Entanglement, being considered as the resource of quantum information science, has been utilized to investigate various properties of condensed matter systems [1,2]. However, it has been discovered that entanglement is not the only kind of useful nonclassical correlation present in quantum systems. ...
... Entanglement, being considered as the resource of quantum information science, has been utilized to investigate various properties of condensed matter systems [1,2]. However, it has been discovered that entanglement is not the only kind of useful nonclassical correlation present in quantum systems. ...
Student Text, pp. 360-364
... elementary charge would be the charge on an individual electron. He assumed, further, that when tiny oil drops are sprayed in a fine mist from an atomizer, they become electrically charged by friction, some acquiring an excess of a few electrons, others acquiring a deficit. Although there was no way ...
... elementary charge would be the charge on an individual electron. He assumed, further, that when tiny oil drops are sprayed in a fine mist from an atomizer, they become electrically charged by friction, some acquiring an excess of a few electrons, others acquiring a deficit. Although there was no way ...
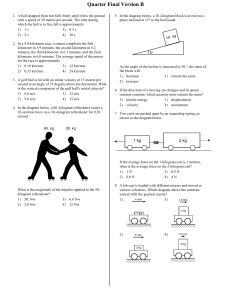
Quarter Final Version B
... 43. According to the Standard Model, a proton is constructed of two up quarks and one down quark (uud) and a neutron is constructed of one up quark and two down quarks (udd). During beta decay, a neutron decays into a proton, an electron, and an electron antineutrino. During this process there is a ...
... 43. According to the Standard Model, a proton is constructed of two up quarks and one down quark (uud) and a neutron is constructed of one up quark and two down quarks (udd). During beta decay, a neutron decays into a proton, an electron, and an electron antineutrino. During this process there is a ...
10 4.0 g of magnesium oxide was found to contain 2.4 g of
... Mass is never lost or gained in chemical reactions. We say that mass is always conserved. In other words, the total mass of products at the end of the reaction is equal to the total mass of the reactants at the beginning. This is because no atoms are created or destroyed during chemical reactions. T ...
... Mass is never lost or gained in chemical reactions. We say that mass is always conserved. In other words, the total mass of products at the end of the reaction is equal to the total mass of the reactants at the beginning. This is because no atoms are created or destroyed during chemical reactions. T ...
Solutions to Problems: Work and Energy
... 76. A simple pendulum consists of a small object of mass m (the bob) suspended by a cord of length L (Fig. 7-33) of negligible mass. A force is applied in the horizontal direction, moving the bob very slowly so the acceleration is essentially zero. (Note that the magnitude of the force needed to var ...
... 76. A simple pendulum consists of a small object of mass m (the bob) suspended by a cord of length L (Fig. 7-33) of negligible mass. A force is applied in the horizontal direction, moving the bob very slowly so the acceleration is essentially zero. (Note that the magnitude of the force needed to var ...
the principle quantum number
... • Map to determine location of the electrons….. • (Methods for denoting earrangement for an atom: orbital notation) ...
... • Map to determine location of the electrons….. • (Methods for denoting earrangement for an atom: orbital notation) ...
problems
... (c) Assume now that the ball is caught at the same height as it was hit: i. How far from home plate does the center fielder need to stand? ii. How much time does he have to position himself? 2. A 5-kg mass object moves under the influence of a force F = (4t2 ı̂ − 3t̂) N, where t is the time in seco ...
... (c) Assume now that the ball is caught at the same height as it was hit: i. How far from home plate does the center fielder need to stand? ii. How much time does he have to position himself? 2. A 5-kg mass object moves under the influence of a force F = (4t2 ı̂ − 3t̂) N, where t is the time in seco ...
SYSTEMS OF PARTICLES AND ROTATIONAL MOTION
... mass and all the external forces were applied at that point. ...
... mass and all the external forces were applied at that point. ...
Chapter 3 Stoichiometry: Calculations with Chemical Formulas and
... Formula units for ionic compounds are equal to their empirical formulas. To determine the EF, given mass % or masses of each element in a compound: 1. Convert masses to moles. 2. Find the mole ratio (i.e. divide by the lowest number of moles). 3. Make sure it is a whole number ratio. One can calcula ...
... Formula units for ionic compounds are equal to their empirical formulas. To determine the EF, given mass % or masses of each element in a compound: 1. Convert masses to moles. 2. Find the mole ratio (i.e. divide by the lowest number of moles). 3. Make sure it is a whole number ratio. One can calcula ...
4.6 Oxidation-Reduction (Redox) Reactions Oxidation Reduction
... must gain those electrons (be reduced). B. Reducing Agent- the substance that causes reduction to occur. loses one or more electrons and undergoes oxidation oxidation # of atom increases metals act as good reducing agents [ Na (s) , Fe (s) , Ca (s)] - Group 1A metals give up one electron to become m ...
... must gain those electrons (be reduced). B. Reducing Agent- the substance that causes reduction to occur. loses one or more electrons and undergoes oxidation oxidation # of atom increases metals act as good reducing agents [ Na (s) , Fe (s) , Ca (s)] - Group 1A metals give up one electron to become m ...
Lecture 2 - Chemistry at Winthrop University
... equation so that the Law of Conservation of Matter is not violated •6 molecules of Cl2 react with 1 molecule of P4 •3 molecules of Cl2 react with 2 molecules of Fe ...
... equation so that the Law of Conservation of Matter is not violated •6 molecules of Cl2 react with 1 molecule of P4 •3 molecules of Cl2 react with 2 molecules of Fe ...
The Electric Force
... Determine the initial launch velocity of the proton when it is very far from the deuterium by equating the work down by the electric force to the change in the proton’s kinetic energy. Assume the proton travels directly toward the deuterium and momentarily “stops” at the distance of closest approach ...
... Determine the initial launch velocity of the proton when it is very far from the deuterium by equating the work down by the electric force to the change in the proton’s kinetic energy. Assume the proton travels directly toward the deuterium and momentarily “stops” at the distance of closest approach ...
Chapter 2: Interacting Rydberg atoms
... electric fields, with their polarizability scaling with the principal quantum number like n∗ 7 . This is also the case when the electric field is generated by the charge distribution of the Rydberg electron of another atom, therefore we can expect Rydberg atoms to exhibit very strong interactions. T ...
... electric fields, with their polarizability scaling with the principal quantum number like n∗ 7 . This is also the case when the electric field is generated by the charge distribution of the Rydberg electron of another atom, therefore we can expect Rydberg atoms to exhibit very strong interactions. T ...
C:\BOB\HSC\Exams 05\Supps\Physics 3204 August 2005 no
... 52.(f) An electron travels in a path perpendicular to a 1.00 × 10-3 T magnetic field. If the radius of the circular path is 2.0 cm, how fast must the electron be travelling? ...
... 52.(f) An electron travels in a path perpendicular to a 1.00 × 10-3 T magnetic field. If the radius of the circular path is 2.0 cm, how fast must the electron be travelling? ...
Topic 20 specification content - A
... I can use 1H NMR and 13C NMR spectra and chemical shift data from the Chemistry Data Booklet to suggest possible structures or part structures for molecules, use integration data from 1H NMR spectra to determine the relative numbers of equivalent protons in the molecule and use the n+1 rule to deduc ...
... I can use 1H NMR and 13C NMR spectra and chemical shift data from the Chemistry Data Booklet to suggest possible structures or part structures for molecules, use integration data from 1H NMR spectra to determine the relative numbers of equivalent protons in the molecule and use the n+1 rule to deduc ...
work book 1-3
... 15. The sublevel 4d is filled with electrons just before 6s sublevel. 16. The total number of electrons in (s) orbitals in germanium atom (Ge32) is 15. 17. (2 , 6) is the distribution of electrons in oxygen atom according to Hund’s rule. ...
... 15. The sublevel 4d is filled with electrons just before 6s sublevel. 16. The total number of electrons in (s) orbitals in germanium atom (Ge32) is 15. 17. (2 , 6) is the distribution of electrons in oxygen atom according to Hund’s rule. ...
Chemistry - Resonance
... The main reasons for this huge number of organic compounds are (i) Catenation : The property of self linking of carbon atoms through covalent bonds to form long straight or branched chains and rings of different sizes is called catenation.Carbon shows maximum catenation in the periodic table due to ...
... The main reasons for this huge number of organic compounds are (i) Catenation : The property of self linking of carbon atoms through covalent bonds to form long straight or branched chains and rings of different sizes is called catenation.Carbon shows maximum catenation in the periodic table due to ...
Chapter 2 Matter and Change
... – cannot be broken down any simpler and still have properties of that element! – all one kind of atom. Compounds are substances that can be broken down only by chemical methods – when broken down, the pieces have completely different properties than the original compound. – made of two or more atoms ...
... – cannot be broken down any simpler and still have properties of that element! – all one kind of atom. Compounds are substances that can be broken down only by chemical methods – when broken down, the pieces have completely different properties than the original compound. – made of two or more atoms ...
Atomic Physics
... neutral, it also has 3 electrons. Bohr’s model is not applicable. If two electrons are stripped away, one ends up with the ion Li2+ . What is the ionization energy of Li2+ ? Solution: Since one has only one electron left, the Bohr’s model can be used. The ionization energy is found by putting n = 1 ...
... neutral, it also has 3 electrons. Bohr’s model is not applicable. If two electrons are stripped away, one ends up with the ion Li2+ . What is the ionization energy of Li2+ ? Solution: Since one has only one electron left, the Bohr’s model can be used. The ionization energy is found by putting n = 1 ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.