Read more - Hans Laroo
... It all started with the perceived lack of ability to produce precise very low current and very high resistance input in order to measure low values of current (nano and pico meters) and high input ...
... It all started with the perceived lack of ability to produce precise very low current and very high resistance input in order to measure low values of current (nano and pico meters) and high input ...
File
... A) Atoms are neutral because they contain the same number of protons and electrons B) All atoms of a given element must contain the same number protons, electrons, and neutrons. C) Most of the volume of an atom contains only electrons D) The nucleus is positively charged E) Almost all of the mass of ...
... A) Atoms are neutral because they contain the same number of protons and electrons B) All atoms of a given element must contain the same number protons, electrons, and neutrons. C) Most of the volume of an atom contains only electrons D) The nucleus is positively charged E) Almost all of the mass of ...
CHEM 1405 Practice Exam #2 (2015)
... A) Solid sodium carbonate is heated to give solid sodium oxide and carbon dioxide gas. B) Sodium carbonate decomposes to sodium oxide and carbon dioxide. C) Sodium carbonate decomposes to sodium oxide and carbon dioxide gas. D) Sodium carbonate is heated to give sodium oxide and carbon dioxide. 20) ...
... A) Solid sodium carbonate is heated to give solid sodium oxide and carbon dioxide gas. B) Sodium carbonate decomposes to sodium oxide and carbon dioxide. C) Sodium carbonate decomposes to sodium oxide and carbon dioxide gas. D) Sodium carbonate is heated to give sodium oxide and carbon dioxide. 20) ...
Teaching Modern Physics - IMSA Digital Commons
... AP Physics B contains 10% atomic and nuclear structure – which means it has less quantum mechanics than AP Chemistry (20%) Serway’s book spends about a sixth of the book on modern physics (often skipped, since it is at the end) NGSS has one relevant standard: ...
... AP Physics B contains 10% atomic and nuclear structure – which means it has less quantum mechanics than AP Chemistry (20%) Serway’s book spends about a sixth of the book on modern physics (often skipped, since it is at the end) NGSS has one relevant standard: ...
... Thi s cou r s e pr ovide s an introduction to the hi s torica l dev elopment o f modern p hysics a nd t o t h e Schroedinger f ormu l at i on of quantum mechan i c s . Specific topic s will inc lude s quare well p o t en t ial s b arri e r s , th e s i mp le h armonic o s cil l at or potentia l and ...
Chemical and physical changes
... move more or less quickly and they come close together or move away. In chemical reactions, molecules change because some substances disappear and other new ones appear. The molecular kinetic theory explains chemical changes supposing that molecules break when they hit to each other and the resultin ...
... move more or less quickly and they come close together or move away. In chemical reactions, molecules change because some substances disappear and other new ones appear. The molecular kinetic theory explains chemical changes supposing that molecules break when they hit to each other and the resultin ...
AP Chapter Five Outline
... Oxidation numbers compare the charge of an uncombined atom with its actual charge in a compound. All neutral atoms have an equal number of protons and electrons and thus have no net charge. Oxidation numbers of atoms in molecular compound are assigned as though electrons were completely transfer ...
... Oxidation numbers compare the charge of an uncombined atom with its actual charge in a compound. All neutral atoms have an equal number of protons and electrons and thus have no net charge. Oxidation numbers of atoms in molecular compound are assigned as though electrons were completely transfer ...
- IMSA Digital Commons
... AP Physics B contains 10% atomic and nuclear structure – which means it has less quantum mechanics than AP Chemistry (20%) Serway’s book spends about a sixth of the book on modern physics (often skipped, since it is at the end) NGSS has one relevant standard: HS-PS4-3: ...
... AP Physics B contains 10% atomic and nuclear structure – which means it has less quantum mechanics than AP Chemistry (20%) Serway’s book spends about a sixth of the book on modern physics (often skipped, since it is at the end) NGSS has one relevant standard: HS-PS4-3: ...
Wednesday, Feb. 25, 2015
... – Covers from CH1.1 through what we learn March 2 plus the math refresher in the appendices – Mid-term exam constitutes 20% of the total – Please do NOT miss the exam! You will get an F if you miss it. – BYOF: You may bring a one 8.5x11.5 sheet (front and back) of handwritten formulae and values of ...
... – Covers from CH1.1 through what we learn March 2 plus the math refresher in the appendices – Mid-term exam constitutes 20% of the total – Please do NOT miss the exam! You will get an F if you miss it. – BYOF: You may bring a one 8.5x11.5 sheet (front and back) of handwritten formulae and values of ...
Chapter 30: Quantum Physics
... Calculate the energy of a single photon from its wavelength using equations 30-4 and 14-1. Divide the total energy by the energy of the single photon to calculate the number of photons. 1. (a) Use equations 30-4 and 14-1 to write the energy of a single photon: 2. Divide the total energy by the energ ...
... Calculate the energy of a single photon from its wavelength using equations 30-4 and 14-1. Divide the total energy by the energy of the single photon to calculate the number of photons. 1. (a) Use equations 30-4 and 14-1 to write the energy of a single photon: 2. Divide the total energy by the energ ...
Slide 1 - Western Engineering
... propane at 1 bar, 273 K. How much air must be supplied (at these conditions)? • Solution: (a) Need mO2 required to burn 1 m3 C3H8 (propane) – To accomplish this task we must first determine the relative amount of reactants and products to burn the propane. This requires setting up the chemical react ...
... propane at 1 bar, 273 K. How much air must be supplied (at these conditions)? • Solution: (a) Need mO2 required to burn 1 m3 C3H8 (propane) – To accomplish this task we must first determine the relative amount of reactants and products to burn the propane. This requires setting up the chemical react ...
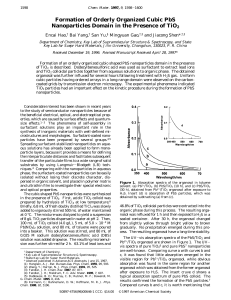
Formation of Orderly Organized Cubic PbS Nanoparticles Domain in
... reactive time was prolonged. This indicated that a slow crystallite growth occurred for a longer reactive time. Samples for transmission electron microscopy (TEM) were prepared as follows. The nanoparticles-containing toluene solution was dropped on the surface of water, which dispersed to be a laye ...
... reactive time was prolonged. This indicated that a slow crystallite growth occurred for a longer reactive time. Samples for transmission electron microscopy (TEM) were prepared as follows. The nanoparticles-containing toluene solution was dropped on the surface of water, which dispersed to be a laye ...
Nessun titolo diapositiva
... Computer simulations of Molecular systems: accuracy of molecular model and force field Four points to consider: 1) Classical mechanics of point masses: the position of one particle depends on the positions of the others through the effective interaction function 2) System size and number of degrees ...
... Computer simulations of Molecular systems: accuracy of molecular model and force field Four points to consider: 1) Classical mechanics of point masses: the position of one particle depends on the positions of the others through the effective interaction function 2) System size and number of degrees ...
Newton`s 2nd Law
... #1) Plowing into the snow - this is akin to air resistance - except that one might sink further into the snow complicating the matter #2) Regelation - with snow the snow will melt with a heavier weight creating a water barrier that reduces friction! But if you are under a certain weight (really pre ...
... #1) Plowing into the snow - this is akin to air resistance - except that one might sink further into the snow complicating the matter #2) Regelation - with snow the snow will melt with a heavier weight creating a water barrier that reduces friction! But if you are under a certain weight (really pre ...
ElectrostaticsI
... Charging by induction in 4 steps: Steps 3 and 4 3. While the plastic rod is nearby, you touch one end of a conducting wire to the right surface of the ball and the other end to the ground. 4. Now disconnect the wire, and then remove the rod. A net positive charge is left on the ball. The earth acqu ...
... Charging by induction in 4 steps: Steps 3 and 4 3. While the plastic rod is nearby, you touch one end of a conducting wire to the right surface of the ball and the other end to the ground. 4. Now disconnect the wire, and then remove the rod. A net positive charge is left on the ball. The earth acqu ...
Electric field of a point charge
... Charging by induction in 4 steps: Steps 3 and 4 3. While the plastic rod is nearby, you touch one end of a conducting wire to the right surface of the ball and the other end to the ground. 4. Now disconnect the wire, and then remove the rod. A net positive charge is left on the ball. The earth acqu ...
... Charging by induction in 4 steps: Steps 3 and 4 3. While the plastic rod is nearby, you touch one end of a conducting wire to the right surface of the ball and the other end to the ground. 4. Now disconnect the wire, and then remove the rod. A net positive charge is left on the ball. The earth acqu ...
Chapter 20 Atomic Spectroscopy
... Usually make calibration curve by dissolving a standard in water In method of standard addition make standard curve by adding measured amounts of analyte to sample (see figure 20-17), and then extrapolate back to the zero point, where you haven’t added anything Why does this work? When we make a sta ...
... Usually make calibration curve by dissolving a standard in water In method of standard addition make standard curve by adding measured amounts of analyte to sample (see figure 20-17), and then extrapolate back to the zero point, where you haven’t added anything Why does this work? When we make a sta ...
Chemistry 11 – Course Review
... Bohr came up with an atomic model to explain the spectrum of ______________________. He said that the atom has certain _______________ levels which are allowed. These levels corresponded to ____________________ in which electrons move. If an electron absorbs a certain photon of energy, it will jump ...
... Bohr came up with an atomic model to explain the spectrum of ______________________. He said that the atom has certain _______________ levels which are allowed. These levels corresponded to ____________________ in which electrons move. If an electron absorbs a certain photon of energy, it will jump ...
Proper time. Announcements Today`s class Conservation of
... This definition of the relativistic mass-energy E fulfills our condition of conservation of total energy. (Not proven here, but we shall see several examples where this proves to be correct.) ...
... This definition of the relativistic mass-energy E fulfills our condition of conservation of total energy. (Not proven here, but we shall see several examples where this proves to be correct.) ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.