
Chapter Six: The Structure of the atoms
... The quantum number (n) defines the energies of the allowed orbits in the H atom. The energy of an electron in an orbit has a negative value because the electron in the atom has a lower energy than when it is free. The zero of energy occurs when n = (when the electron is infinitely separated from t ...
... The quantum number (n) defines the energies of the allowed orbits in the H atom. The energy of an electron in an orbit has a negative value because the electron in the atom has a lower energy than when it is free. The zero of energy occurs when n = (when the electron is infinitely separated from t ...
Electric Charge
... Charging By Induction • Bringing a positively charged rod close to, but not touching, a neutral conducting sphere will cause the negative charges within the neutral sphere to be attracted to the positively charged rod and the positive charges within the neutral sphere to be repelled to the opposite ...
... Charging By Induction • Bringing a positively charged rod close to, but not touching, a neutral conducting sphere will cause the negative charges within the neutral sphere to be attracted to the positively charged rod and the positive charges within the neutral sphere to be repelled to the opposite ...
study guide - Mechanical and Nuclear Engineering
... 9. Explain the signi cance of wave-particle duality. 10. Describe how the Davisson-Germer electron scattering experiment con rmed the wave-nature of electrons. 11. Describe the basic idea used by Schrodinger to derive his famous wave equation. 12. Explain the important consequences of Schrodinger' ...
... 9. Explain the signi cance of wave-particle duality. 10. Describe how the Davisson-Germer electron scattering experiment con rmed the wave-nature of electrons. 11. Describe the basic idea used by Schrodinger to derive his famous wave equation. 12. Explain the important consequences of Schrodinger' ...
Solution Preparation Final Goueth
... 64. Which is true about equal volumes of CH4 and O2 at 20 °C and 1 atm pressure? (A) The CH4 sample has a mass that is one-half that of the O2 sample. (B) The number of O2 molecules is twice as large as the number of CH4 molecules. (C) The average kinetic energy of the O2 molecules is one-half that ...
... 64. Which is true about equal volumes of CH4 and O2 at 20 °C and 1 atm pressure? (A) The CH4 sample has a mass that is one-half that of the O2 sample. (B) The number of O2 molecules is twice as large as the number of CH4 molecules. (C) The average kinetic energy of the O2 molecules is one-half that ...
UNIT 7 – CHEMICAL REACTIONS
... 3. Evidence of a chemical reaction can come in the form of things you can see, smell and hear. But, this does not mean a reaction has actually taken place. To verify a reaction has occurred one must analyze the products to verify their physical and chemical properties. 4. Chemical reactions release ...
... 3. Evidence of a chemical reaction can come in the form of things you can see, smell and hear. But, this does not mean a reaction has actually taken place. To verify a reaction has occurred one must analyze the products to verify their physical and chemical properties. 4. Chemical reactions release ...
Moles Level
... element there are Multiply # of atoms by that element’s atomic mass Add all atoms’ atomic masses together ...
... element there are Multiply # of atoms by that element’s atomic mass Add all atoms’ atomic masses together ...
First lecture, 7.10.03
... givethese up allboth knowledge Copenhagen:or noknow waveSx function hasup both properties defined – and give all those knowledge of Sz... and the wave function is all you can possibly know. EPR are cheating, discussing measurements they didn’t do. ...
... givethese up allboth knowledge Copenhagen:or noknow waveSx function hasup both properties defined – and give all those knowledge of Sz... and the wave function is all you can possibly know. EPR are cheating, discussing measurements they didn’t do. ...
Exploring the quantum world
... as each electron arrived at the photographic plate). Instead, Born postulated that Schrodinger’s equation described the probability of an electron (or particle) being in a given location while behaving as a wave. An electron could be here, there, or perhaps over there, and the probabilities of each ...
... as each electron arrived at the photographic plate). Instead, Born postulated that Schrodinger’s equation described the probability of an electron (or particle) being in a given location while behaving as a wave. An electron could be here, there, or perhaps over there, and the probabilities of each ...
doc - StealthSkater
... What TGD could say about this. 1. In TGD framework, one has many-sheeted space-time; dark matter hierarchy represented by the book-like structure of the generalized imbedding space; and dark energy is replaced with dark matter at pages of the book with gigantic Planck constant so that the Compton le ...
... What TGD could say about this. 1. In TGD framework, one has many-sheeted space-time; dark matter hierarchy represented by the book-like structure of the generalized imbedding space; and dark energy is replaced with dark matter at pages of the book with gigantic Planck constant so that the Compton le ...
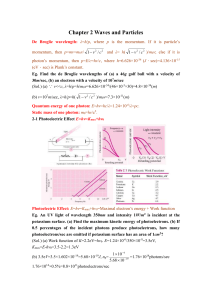
Chapter 2 Waves and Particles De Broglie wavelength: λ=h/p, where
... (eV.sec) is Plank’s constant. Eg. Find the de Broglie wavelengths of (a) a 46g golf ball with a velocity of 30m/sec, (b) an electron with a velocity of 107m/sec (Sol.) (a) ∵ v<
... (eV.sec) is Plank’s constant. Eg. Find the de Broglie wavelengths of (a) a 46g golf ball with a velocity of 30m/sec, (b) an electron with a velocity of 107m/sec (Sol.) (a) ∵ v<
Equations - Pearson Schools and FE Colleges
... • In word equations, use only the chemical names of the substances • In chemical equations, correctly write numbers in formulae, e.g. H2O, not H2O or H2O • When balancing, do not change any chemical formula. Only put numbers in front of a formula. ...
... • In word equations, use only the chemical names of the substances • In chemical equations, correctly write numbers in formulae, e.g. H2O, not H2O or H2O • When balancing, do not change any chemical formula. Only put numbers in front of a formula. ...
Multiple choice questions [60 points]
... The graph shows a plot of the gravitational potential energy U of a 1kg body as a function of its height h above the surface of a planet. The acceleration due to gravity at the surface of the planet is A. B. C. D. ...
... The graph shows a plot of the gravitational potential energy U of a 1kg body as a function of its height h above the surface of a planet. The acceleration due to gravity at the surface of the planet is A. B. C. D. ...
Chemistry Packet: Chemical Bonding
... 4) The chemical formula for a molecular compound is called a_________________. 5) _______________________________= a shorthand representation showing the types and numbers of atoms combined in a single molecule a) Ex: hydrogen peroxide; H2O2;________ atoms of hydrogen and _______ atoms of oxygen ar ...
... 4) The chemical formula for a molecular compound is called a_________________. 5) _______________________________= a shorthand representation showing the types and numbers of atoms combined in a single molecule a) Ex: hydrogen peroxide; H2O2;________ atoms of hydrogen and _______ atoms of oxygen ar ...
File
... The value of G°f for formation of Cr2O3 is –540 kJ mol–1 and that of Al2O3 is – 827 kJ mol–1. Is the reduction of Cr2O3 possible with aluminium? ...
... The value of G°f for formation of Cr2O3 is –540 kJ mol–1 and that of Al2O3 is – 827 kJ mol–1. Is the reduction of Cr2O3 possible with aluminium? ...
Period 5 Activity Sheet: Gravity, Mass and Weight
... Balance a meter stick on two fingers. Start with one finger under each end of the meter stick. Slowly slide your fingers together while balancing the meter stick on them. Explain what happens to your fingers in terms of the downward force of the stick on your finger, the friction between the stick a ...
... Balance a meter stick on two fingers. Start with one finger under each end of the meter stick. Slowly slide your fingers together while balancing the meter stick on them. Explain what happens to your fingers in terms of the downward force of the stick on your finger, the friction between the stick a ...
Chapter 6 Chemical Reactions: An Introduction
... • Shorthand way of describing a reaction • Provides information about the reaction: – Formulas of reactants and products – States of reactants and products – Relative numbers of reactant and product molecules that are required – Can be used to determine weights of reactants used and of products that ...
... • Shorthand way of describing a reaction • Provides information about the reaction: – Formulas of reactants and products – States of reactants and products – Relative numbers of reactant and product molecules that are required – Can be used to determine weights of reactants used and of products that ...
2018 Specimen Paper 2 - Cambridge International Examinations
... After the copper(II) sulfate had crystallised the students dried and weighed the crystals. Which student produced the highest percentage yield of hydrated copper(II) sulfate? mass of copper(II) oxide used / g ...
... After the copper(II) sulfate had crystallised the students dried and weighed the crystals. Which student produced the highest percentage yield of hydrated copper(II) sulfate? mass of copper(II) oxide used / g ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.














![Multiple choice questions [60 points]](http://s1.studyres.com/store/data/002785313_1-b2734444f348f25d9b46ea15f542520b-300x300.png)






