jyvaskla2 - School of Chemistry
... A method of obtaining chemically significant information from the electron density (rho) is a quantum-mechanical observable, and may also be obtained from experiment ...
... A method of obtaining chemically significant information from the electron density (rho) is a quantum-mechanical observable, and may also be obtained from experiment ...
B) Examples of Avagadro`s Number
... 1) Remember when calculating atomic mass, we use amu (atomic mass units) 1amu = 1.66 x 10-24g. 2) The opposite of that equality is 1g = 6.02214 x 1023 amu. 3) That means that if you take 6.02214 x 1023 protons (or neutrons), they would equal 1g. 4) So, 6.02214 x 1023 carbon atoms would weigh (6.0221 ...
... 1) Remember when calculating atomic mass, we use amu (atomic mass units) 1amu = 1.66 x 10-24g. 2) The opposite of that equality is 1g = 6.02214 x 1023 amu. 3) That means that if you take 6.02214 x 1023 protons (or neutrons), they would equal 1g. 4) So, 6.02214 x 1023 carbon atoms would weigh (6.0221 ...
Slide 1 of 24
... Hindenburg erupted into a fireball. Within a short time, 210,000 cubic meters of hydrogen had burned and the airship was destroyed. The chemical reaction that occurred is “hydrogen combines with oxygen to produce water.” You will learn to represent this chemical reaction by a chemical equation. Slid ...
... Hindenburg erupted into a fireball. Within a short time, 210,000 cubic meters of hydrogen had burned and the airship was destroyed. The chemical reaction that occurred is “hydrogen combines with oxygen to produce water.” You will learn to represent this chemical reaction by a chemical equation. Slid ...
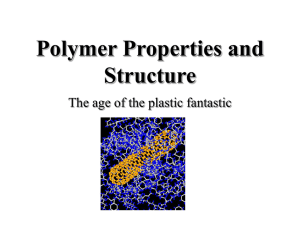
Polymer Properties and Structure
... Name three steps in the addition polymerization process Distinguish between addition and condensation polymerization Describe essential features of condensation polymerization Identify polymerization process used on basis of polymer composition ...
... Name three steps in the addition polymerization process Distinguish between addition and condensation polymerization Describe essential features of condensation polymerization Identify polymerization process used on basis of polymer composition ...
Simulation of discharging dust grains by laser excitation of neutral...
... The charge on a dust grain in a plasma depends1,2 on the size of the grain and the parameters of the plasma: electron temperature, ion temperature, mass of the ion, the ion and electron masses, and the local ion and electron plasma densities. However, the confinement3,4 and transport of a charged du ...
... The charge on a dust grain in a plasma depends1,2 on the size of the grain and the parameters of the plasma: electron temperature, ion temperature, mass of the ion, the ion and electron masses, and the local ion and electron plasma densities. However, the confinement3,4 and transport of a charged du ...
Electron shell contributions to gamma
... for He and Ar, respectively. As the annihilation γ -ray spectra are symmetric, w(−ε) = w(ε), only positive photon energies (ε > 0 keV) are shown in figure 1. All spectra are normalized to unity at ε = 0. It is seen in figure 1 that in the PW approximation, the momentum distributions of the atomic el ...
... for He and Ar, respectively. As the annihilation γ -ray spectra are symmetric, w(−ε) = w(ε), only positive photon energies (ε > 0 keV) are shown in figure 1. All spectra are normalized to unity at ε = 0. It is seen in figure 1 that in the PW approximation, the momentum distributions of the atomic el ...
PPT
... 4: If the potential U(x) has a center of symmetry (such as the center of the well above), the eigenstates will be, alternately, even and odd functions about that center of symmetry. Lecture 12, p 3 ...
... 4: If the potential U(x) has a center of symmetry (such as the center of the well above), the eigenstates will be, alternately, even and odd functions about that center of symmetry. Lecture 12, p 3 ...
Oxidation-Reduction Reactions
... chemical compound changes. Some common redox reactions include fire, rusting of metals, browning of fruit, and photosynthesis. In simpler terms, redox reactions involve the transfer of electrons from one substance to another. In a redox reaction, electrons can never be “lost”; if one substance loses ...
... chemical compound changes. Some common redox reactions include fire, rusting of metals, browning of fruit, and photosynthesis. In simpler terms, redox reactions involve the transfer of electrons from one substance to another. In a redox reaction, electrons can never be “lost”; if one substance loses ...
Quantum impurity problem in ultracold gases: Dimitri M Gangardt Alex Kamenev,
... Quantum impurity problem in ultracold gases: from dark solitons to quantum ferromagnets ...
... Quantum impurity problem in ultracold gases: from dark solitons to quantum ferromagnets ...
De Broglie Wavelets versus Schrodinger Wave Functions
... ballistic classical trajectory. In addition to such a localized de Broglie wavelet, there is another Schrodinger-type delocalized wave carrier moving at a phase velocity shown to be c2/v. These results agree with earlier beliefs of Einstein who introduced photons as localized lumps of electromagneti ...
... ballistic classical trajectory. In addition to such a localized de Broglie wavelet, there is another Schrodinger-type delocalized wave carrier moving at a phase velocity shown to be c2/v. These results agree with earlier beliefs of Einstein who introduced photons as localized lumps of electromagneti ...
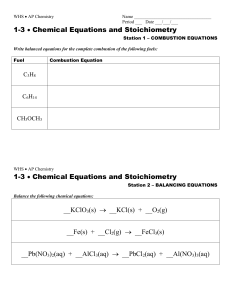
Chemical Equations and Reaction Types Lab
... 3) Determine the products and write the correct formula for each product. Once the correct formula is written it must not be changed during the subsequent balancing operation. 4) Balance the chemical equation. Do NOT change any chemical formulas while balancing. a) Choose the compound with the great ...
... 3) Determine the products and write the correct formula for each product. Once the correct formula is written it must not be changed during the subsequent balancing operation. 4) Balance the chemical equation. Do NOT change any chemical formulas while balancing. a) Choose the compound with the great ...
Spectroscopy
... • Excited molecules are subjected to collisions and give up energy down to lower vibrational levels. If it survives long enough it may undergo spontaneous emission and emit remaining energy as radiation in going to the lower electronic state. The spectra has the vibrational spectra of the lower stat ...
... • Excited molecules are subjected to collisions and give up energy down to lower vibrational levels. If it survives long enough it may undergo spontaneous emission and emit remaining energy as radiation in going to the lower electronic state. The spectra has the vibrational spectra of the lower stat ...
Here
... Reacting Masses / Gas Calculations The steps involved in a calculation are as follows : (a) Convert the information given to moles of one substance. (b) Use the chemical equation to find moles of other substance needed. (c) Convert back from moles to mass (or concentration, volume etc.) ...
... Reacting Masses / Gas Calculations The steps involved in a calculation are as follows : (a) Convert the information given to moles of one substance. (b) Use the chemical equation to find moles of other substance needed. (c) Convert back from moles to mass (or concentration, volume etc.) ...
Ch. 3 9-Station Review
... Hydrogen gas was generated according to the equation: Zn(s) + 2HCl(aq) H2(g) + ZnCl2(aq) When 25.00 grams of Zn metal reacted with excess HCl 7.50 L H2(g) was collected at STP. The theoretical yield of H2(g) for this reaction is: (show work) ...
... Hydrogen gas was generated according to the equation: Zn(s) + 2HCl(aq) H2(g) + ZnCl2(aq) When 25.00 grams of Zn metal reacted with excess HCl 7.50 L H2(g) was collected at STP. The theoretical yield of H2(g) for this reaction is: (show work) ...
of THE by 0.
... effects of stray electric and magnetic fields and to subtract such effects from their results. ...
... effects of stray electric and magnetic fields and to subtract such effects from their results. ...
Coupling MOS Quantum Dot and Phosphorus Donor Qubit Systems
... [1] F. Zwanenburg et al. "Silicon quantum electronics,” Review of Modern. ...
... [1] F. Zwanenburg et al. "Silicon quantum electronics,” Review of Modern. ...
AP Chemistry - Chagrin Falls Schools
... planned absence you are required to hand in the assignment to your teacher on the assigned date. Failure to do so will result in an enforcement of the aforementioned late policies. Also, if a long term project was assigned and you were either absent the day it was due or days leading up to the assig ...
... planned absence you are required to hand in the assignment to your teacher on the assigned date. Failure to do so will result in an enforcement of the aforementioned late policies. Also, if a long term project was assigned and you were either absent the day it was due or days leading up to the assig ...
Name
... Points A, B, C, and D in the diagram below represent positions of the ball as it falls. At which position will the ball have the greatest kinetic energy? (1) A (3) C (2) B (4) D Part 3: Interpreting experimental results Read the text below. Use information from the text to help you answer questions ...
... Points A, B, C, and D in the diagram below represent positions of the ball as it falls. At which position will the ball have the greatest kinetic energy? (1) A (3) C (2) B (4) D Part 3: Interpreting experimental results Read the text below. Use information from the text to help you answer questions ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.