4 Unit Packet - SRHSchem
... 3. Is it possible, given the original data in Table 1, to determine the % composition by mass of H for 2-butene without using the equation given in the model? If so, how? ...
... 3. Is it possible, given the original data in Table 1, to determine the % composition by mass of H for 2-butene without using the equation given in the model? If so, how? ...
odd - WWW2
... kinetic factors (that is, comparative activation energies) can lead to other products. ...
... kinetic factors (that is, comparative activation energies) can lead to other products. ...
Version 001 – Term B Final Review – tubman – (20141B) 1 This
... 4. The charge resides uniformly throughout the sphere. 5. The electric field inside the sphere is constant in magnitude, but not zero. Explanation: Every point in the conductor becomes equipotential, and the electric field is defined as the gradient of the electric potential, so inside the conductin ...
... 4. The charge resides uniformly throughout the sphere. 5. The electric field inside the sphere is constant in magnitude, but not zero. Explanation: Every point in the conductor becomes equipotential, and the electric field is defined as the gradient of the electric potential, so inside the conductin ...
COORDIHAflON CHEMISTRY REVIEWS
... Metal cluster chemistry has been widely explored with d metals. Naturally, discrete clusters with the metal atom core surrounded by a ligand shell dominate solution chemistry. In the solid state such clusters may occur as discrete entities or linked via bridging ligands and even condensed by sharing ...
... Metal cluster chemistry has been widely explored with d metals. Naturally, discrete clusters with the metal atom core surrounded by a ligand shell dominate solution chemistry. In the solid state such clusters may occur as discrete entities or linked via bridging ligands and even condensed by sharing ...
Mexico city 2007 - Università degli Studi dell`Insubria
... Usually obtains 60-90% of correlation energy ...
... Usually obtains 60-90% of correlation energy ...
Stoichiometry: Calculations with Chemical Formulas and
... (0.350 mol) and its chemical formula C6H12O6. The unknown is the number of H atoms in the sample. Plan Avogadro’s number provides the conversion factor between number of moles of C6H12O6 and number of molecules of C6H12O6: 1 mol C6H12O6 = 6.02 1023 molecules C6H12O6. Once we know the number of mol ...
... (0.350 mol) and its chemical formula C6H12O6. The unknown is the number of H atoms in the sample. Plan Avogadro’s number provides the conversion factor between number of moles of C6H12O6 and number of molecules of C6H12O6: 1 mol C6H12O6 = 6.02 1023 molecules C6H12O6. Once we know the number of mol ...
Factors affecting the Cl atom density in a chlorine discharge
... properties and the rate coefficients for various electronparticle (atom or molecule) reactions.The EEDF depends on the electric field, neutral gas density, excitation frequency, and plasma gas composition. The latter may be quite different than the feedstockgascomposition,but this complication is us ...
... properties and the rate coefficients for various electronparticle (atom or molecule) reactions.The EEDF depends on the electric field, neutral gas density, excitation frequency, and plasma gas composition. The latter may be quite different than the feedstockgascomposition,but this complication is us ...
Addressing of individual atoms in an optical dipole trap
... results in a Doppler frequency shift of atomic transition with respect to the radiation field frequency. This results in a velocity dependent absorbtion of the light and can be used to slow an atom. In order to qualitatively understand the damping of an atomic velocity in the field of red-detuned co ...
... results in a Doppler frequency shift of atomic transition with respect to the radiation field frequency. This results in a velocity dependent absorbtion of the light and can be used to slow an atom. In order to qualitatively understand the damping of an atomic velocity in the field of red-detuned co ...
Idealization in Quantum Field Theory - Philsci
... concerning the corresponding Type A Theory are also idealizations of the Type B Theory. Furthermore, there are supplementary idealizations in Type B Theories. Here I shall discuss only Type B Theories. There are several ways to detect idealizations in a Type B Theory in practice. Just like in the ca ...
... concerning the corresponding Type A Theory are also idealizations of the Type B Theory. Furthermore, there are supplementary idealizations in Type B Theories. Here I shall discuss only Type B Theories. There are several ways to detect idealizations in a Type B Theory in practice. Just like in the ca ...
slides
... Students develop perspecWves on the physical interpretaWon of QM • Whether instructors akend to them or not • When they do, instrucWon has influence • When not, greater tendency to be intuiWvely real ...
... Students develop perspecWves on the physical interpretaWon of QM • Whether instructors akend to them or not • When they do, instrucWon has influence • When not, greater tendency to be intuiWvely real ...
Name __KEY____________ Per. ______ Polarity and
... For chemical reactions the reactants are found on the ___ left___ (left/ right) side of the chemical equation and the products are found on the __ right__ (left/ right) side of the chemical equation. Two quantities that are always conserved, meaning stays the same, during a chemical reaction are [ci ...
... For chemical reactions the reactants are found on the ___ left___ (left/ right) side of the chemical equation and the products are found on the __ right__ (left/ right) side of the chemical equation. Two quantities that are always conserved, meaning stays the same, during a chemical reaction are [ci ...
Chapter 5: Calculations and the Chemical Equation
... 2. The products which are formed by the reaction. 3. The amounts (moles) of each substance used and each substance produced. The Numbers in a Chemical Equation: 1. Subscripts: The small numbers to the lower right of chemical symbols. Subscripts represent the number of atoms of each element in the mo ...
... 2. The products which are formed by the reaction. 3. The amounts (moles) of each substance used and each substance produced. The Numbers in a Chemical Equation: 1. Subscripts: The small numbers to the lower right of chemical symbols. Subscripts represent the number of atoms of each element in the mo ...
terpconnect.umd.edu
... Rayleigh limit: the equilibrium state at which further addition of charge will cause the drop to become unstable and break ...
... Rayleigh limit: the equilibrium state at which further addition of charge will cause the drop to become unstable and break ...
chemistry 110 lecture
... a) Count and compare the number of atoms of each element on both sides of the equation. b) Balance each element individually by placing whole numbers in front of the chemical formula c) Check all elements after each individual element is balanced to see, whether or not in balancing one element, othe ...
... a) Count and compare the number of atoms of each element on both sides of the equation. b) Balance each element individually by placing whole numbers in front of the chemical formula c) Check all elements after each individual element is balanced to see, whether or not in balancing one element, othe ...
ACTION AT A DISTANCE AND COSMOLOGY: A Historical
... the 12 F (a) fields of all particles a 6= b that have been excited by this total field Ftot for these influences arrive back at B instantaneously. Thus only the future light cone of B comes into play. The reaction from the universe on the future light cone cancels the advanced component of F (b) and ...
... the 12 F (a) fields of all particles a 6= b that have been excited by this total field Ftot for these influences arrive back at B instantaneously. Thus only the future light cone of B comes into play. The reaction from the universe on the future light cone cancels the advanced component of F (b) and ...
GASEOUS IONIZATION AND ION TRANSPORT: An Introduction to
... certainly not understood by the general public. If you were to take a poll of the general population about engineering and science topics, nearly everyone would understand what robotics is and most would likely be able to surmise, on some level, what an aerospace engineer does. In fact, most people ...
... certainly not understood by the general public. If you were to take a poll of the general population about engineering and science topics, nearly everyone would understand what robotics is and most would likely be able to surmise, on some level, what an aerospace engineer does. In fact, most people ...
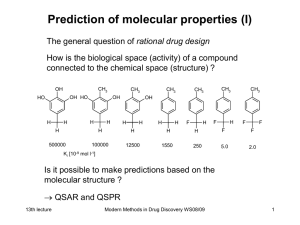
Modern Methods in Drug Discovery
... the movement of the atomic cores from that of the electrons, the so called Born-Oppenheimer Approximation: Atomic cores are > 1000 times heavier than the electrons und thus notice the electrons only as an averaged field ...
... the movement of the atomic cores from that of the electrons, the so called Born-Oppenheimer Approximation: Atomic cores are > 1000 times heavier than the electrons und thus notice the electrons only as an averaged field ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.