Solutions - ChemConnections
... Mass balance indicates that we have the same number and type of atoms on both sides of the equation (so that mass is conserved). Similarly, net charge must also be conserved. We cannot have a buildup of charge on one side of the reaction or the other. In redox reactions, electrons are used to balanc ...
... Mass balance indicates that we have the same number and type of atoms on both sides of the equation (so that mass is conserved). Similarly, net charge must also be conserved. We cannot have a buildup of charge on one side of the reaction or the other. In redox reactions, electrons are used to balanc ...
File - cpprashanths Chemistry
... 2. Question nos. 1 to 8 are very short answer questions and carry 1 mark each. 3. Question nos. 9 to 18 are short answer questions and carry 2 marks each. 4. Question nos. 19 to 27 are also short answer questions and carry 3 marks each 5. Question nos. 28 to 30 are long answer questions and carry 5 ...
... 2. Question nos. 1 to 8 are very short answer questions and carry 1 mark each. 3. Question nos. 9 to 18 are short answer questions and carry 2 marks each. 4. Question nos. 19 to 27 are also short answer questions and carry 3 marks each 5. Question nos. 28 to 30 are long answer questions and carry 5 ...
Chem 12 SM Ch5 Review final new ok revised
... and potential energy, as well as concepts and real world examples of each type of energy. 33. Answers may vary. Students’ diagrams should include what happens to the water molecules and any energy transfers that take place. Ideas could include: The process of liquid water turning into steam is an en ...
... and potential energy, as well as concepts and real world examples of each type of energy. 33. Answers may vary. Students’ diagrams should include what happens to the water molecules and any energy transfers that take place. Ideas could include: The process of liquid water turning into steam is an en ...
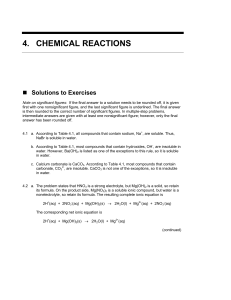
Chapter 4 Solution Manual
... Ions must be present in an aqueous solution for it to conduct an electric current. Ions come from ionic compounds or from other electrolytes such as acids and bases. ...
... Ions must be present in an aqueous solution for it to conduct an electric current. Ions come from ionic compounds or from other electrolytes such as acids and bases. ...
Multiple-choice questions : 1. Which of the following solutions
... In an experiment to determine the concentration of ammonia solution, 25.0 cm3 of the ammonia solution was transferred into a conical flask and titrated against 0.1 M sulphuric acid. A few drops of indicator were added. The titration results are listed in the table below: ...
... In an experiment to determine the concentration of ammonia solution, 25.0 cm3 of the ammonia solution was transferred into a conical flask and titrated against 0.1 M sulphuric acid. A few drops of indicator were added. The titration results are listed in the table below: ...
9278654 PS/Chemistry Ja03 - Dolgeville Central School
... 5 Which event must always occur for a chemical reaction to take place? (1) formation of a precipitate (2) formation of a gas (3) effective collisions between reacting particles (4) addition of a catalyst to the reaction system P.S./Chem.–Jan. ’03 ...
... 5 Which event must always occur for a chemical reaction to take place? (1) formation of a precipitate (2) formation of a gas (3) effective collisions between reacting particles (4) addition of a catalyst to the reaction system P.S./Chem.–Jan. ’03 ...
laman web smk raja perempuan, ipoh
... crystals and their aqueous solutions. 11. calculate enthaphy changes from energy cycles 12. explain qualitatively the influence on solubility of the relationship between enthalphy change of solution, lattice energy of solid, and enthalphy change of hydration, or other ...
... crystals and their aqueous solutions. 11. calculate enthaphy changes from energy cycles 12. explain qualitatively the influence on solubility of the relationship between enthalphy change of solution, lattice energy of solid, and enthalphy change of hydration, or other ...
Chemistry HL Syllabus Details
... often derived from the pseudoscience of alchemy. Lavoisier’s work with oxygen, which overturned the phlogiston theory of heat, could be discussed as an example of a paradigm shift. Int: The discovery of the elements and the arrangement of them is a story that exemplifies how scientific progress is m ...
... often derived from the pseudoscience of alchemy. Lavoisier’s work with oxygen, which overturned the phlogiston theory of heat, could be discussed as an example of a paradigm shift. Int: The discovery of the elements and the arrangement of them is a story that exemplifies how scientific progress is m ...
Science 9 Year End Review The following information includes all
... Name the FOUR factors that can affect the rate of a chemical reaction: Define REACTION RATE Define CATALYST Define INHIBITOR Increasing the _________________, the _________________, and the _________________ _________________ will speed up a reaction. Decreasing these will slow it down. ...
... Name the FOUR factors that can affect the rate of a chemical reaction: Define REACTION RATE Define CATALYST Define INHIBITOR Increasing the _________________, the _________________, and the _________________ _________________ will speed up a reaction. Decreasing these will slow it down. ...
Devillez (ld2653) – Test 1 Review – Devillez – (99998)
... In Rutherford’s gold foil experiment in 1910, α (alpha) particles were fired at gold foil, and the resulting deflection of the particles were observed. Most of the α particles went through the sample undeflected, suggesting that much of the atom was empty space. But of the few α particles that were ...
... In Rutherford’s gold foil experiment in 1910, α (alpha) particles were fired at gold foil, and the resulting deflection of the particles were observed. Most of the α particles went through the sample undeflected, suggesting that much of the atom was empty space. But of the few α particles that were ...
Chemistry Exemplar Problems
... examination is one of the key reasons why other resources and sites of learning are ignored. It further reiterates that the methods used for teaching and evaluation will also determine how effective these textbooks prove for making children’s life at school a happy experience, rather than source of ...
... examination is one of the key reasons why other resources and sites of learning are ignored. It further reiterates that the methods used for teaching and evaluation will also determine how effective these textbooks prove for making children’s life at school a happy experience, rather than source of ...
Energetics
... CH4(g) + 2O2(g) CO2(g) + 2H2O(g) H = -802 kJ mol-1 at 373 K CH4(g) + 2O2(g) CO2(g) + 2H2O(l) H = -890 kJ mol-1 at 298 K ...
... CH4(g) + 2O2(g) CO2(g) + 2H2O(g) H = -802 kJ mol-1 at 373 K CH4(g) + 2O2(g) CO2(g) + 2H2O(l) H = -890 kJ mol-1 at 298 K ...
Alchemist`s Cookbook Student Part 2 (final)
... 4) Use the slider control to increase the number of protons in the nucleus to five instead of one. Then rerun the simulation with an x-velocity of 5 m/s. Describe the results of this simulation and explain why the results are different from those recorded in step #3. ...
... 4) Use the slider control to increase the number of protons in the nucleus to five instead of one. Then rerun the simulation with an x-velocity of 5 m/s. Describe the results of this simulation and explain why the results are different from those recorded in step #3. ...
CHAPTER 1 - THE MOLE SECTION 1
... were able to deduce the mass ratios of the atoms. It did not matter that the first masses obtained were in grams and the second were in pounds. The relative atomic and molecular masses are independent of the units used as long as the units of the two substances being compared are the same. The relat ...
... were able to deduce the mass ratios of the atoms. It did not matter that the first masses obtained were in grams and the second were in pounds. The relative atomic and molecular masses are independent of the units used as long as the units of the two substances being compared are the same. The relat ...
Solutions Manual
... Cellulose is made from repeating units of β-glucose with inversion of every second unit. This produces long, straight chains of cellulose which are linked to each other by hydrogen bonding. In plants, cellulose acts as a structural material. Starch (both amylase and amylopectin) is made from long-ch ...
... Cellulose is made from repeating units of β-glucose with inversion of every second unit. This produces long, straight chains of cellulose which are linked to each other by hydrogen bonding. In plants, cellulose acts as a structural material. Starch (both amylase and amylopectin) is made from long-ch ...
Kinetic isotope effects of 12CH3D+OH and 13CH3D+OH from 278 to
... sink) fractionations is an underdetermined systems (e.g., Pohlman et al., 2009). Recent advances in mass spectrometry (Eiler et al., 2013; Stolper et al., 2014) and laser infrared spectroscopy (Ono et al., 2014; Wang et al., 2015) facilitate measurement of rare doubly substituted isotopologues. The ...
... sink) fractionations is an underdetermined systems (e.g., Pohlman et al., 2009). Recent advances in mass spectrometry (Eiler et al., 2013; Stolper et al., 2014) and laser infrared spectroscopy (Ono et al., 2014; Wang et al., 2015) facilitate measurement of rare doubly substituted isotopologues. The ...
Stoichiometry

Stoichiometry /ˌstɔɪkiˈɒmɨtri/ is the calculation of relative quantities of reactants and products in chemical reactions.Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of product can be empirically determined, then the amount of the other reactants can also be calculated.As seen in the image to the right, where the balanced equation is:CH4 + 2 O2 → CO2 + 2 H2O.Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. Stoichiometry measures these quantitative relationships, and is used to determine the amount of products/reactants that are produced/needed in a given reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry. In the example above, reaction stoichiometry measures the relationship between the methane and oxygen as they react to form carbon dioxide and water.Because of the well known relationship of moles to atomic weights, the ratios that are arrived at by stoichiometry can be used to determine quantities by weight in a reaction described by a balanced equation. This is called composition stoichiometry.Gas stoichiometry deals with reactions involving gases, where the gases are at a known temperature, pressure, and volume and can be assumed to be ideal gases. For gases, the volume ratio is ideally the same by the ideal gas law, but the mass ratio of a single reaction has to be calculated from the molecular masses of the reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the mass ratio.