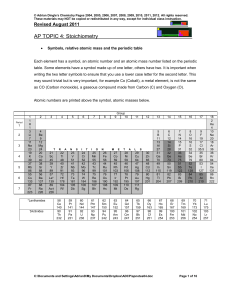
2 Chemical equilibrium occurs when a reaction and its reverse
... 5. 2NO (g) + Cl2 (g) ↔ 2NOCl (g) ...
... 5. 2NO (g) + Cl2 (g) ↔ 2NOCl (g) ...
Unit 6 – The Mole Lesson 1 Moles 1 mole of any substance contains
... ______________________________ = the mass of 1 mole of a substance in grams (also known as Gram Formula Weight or Formula Weight.) Mass given on periodic table is in __________________. Because amu and grams are relative, we can say the masses on the periodic table are in _______. To find __________ ...
... ______________________________ = the mass of 1 mole of a substance in grams (also known as Gram Formula Weight or Formula Weight.) Mass given on periodic table is in __________________. Because amu and grams are relative, we can say the masses on the periodic table are in _______. To find __________ ...
Chemistry I Exam
... The “bright lines” making up the spectra of excited gaseous atoms help to identify the various energy levels of these atoms. Which statement does NOT help to explain the observed line spectra? A. Electrons tend to drop to the lowest available energy levels in an atom. B. Each frequency of light corr ...
... The “bright lines” making up the spectra of excited gaseous atoms help to identify the various energy levels of these atoms. Which statement does NOT help to explain the observed line spectra? A. Electrons tend to drop to the lowest available energy levels in an atom. B. Each frequency of light corr ...
Document
... 4. The oxidation number of hydrogen is +1 except when it is bonded to metals in binary compounds. In these cases, its oxidation number is –1. 5. Group IA metals are +1, IIA metals are +2 and fluorine is always –1. 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to ...
... 4. The oxidation number of hydrogen is +1 except when it is bonded to metals in binary compounds. In these cases, its oxidation number is –1. 5. Group IA metals are +1, IIA metals are +2 and fluorine is always –1. 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to ...
Document
... lead”pencils and as a lubricant for locks, and diamond, the brilliant, hard gemstone. Using the enthalpies of combustion for graphite (-394 kJ/mol) and diamond (-396 kJ/mol), calculate ΔH for the conversion of graphite to diamond: C graphite ( s) Cdiamond ( s ) ...
... lead”pencils and as a lubricant for locks, and diamond, the brilliant, hard gemstone. Using the enthalpies of combustion for graphite (-394 kJ/mol) and diamond (-396 kJ/mol), calculate ΔH for the conversion of graphite to diamond: C graphite ( s) Cdiamond ( s ) ...
Chemical Reactions
... released because atoms that have an attraction for one another are brought back together. Not every bond between atoms in the reactants is necessarily broken during a chemical reaction, but some bonds are. By comparing the energy used when bonds in the reactants are broken with the energy released w ...
... released because atoms that have an attraction for one another are brought back together. Not every bond between atoms in the reactants is necessarily broken during a chemical reaction, but some bonds are. By comparing the energy used when bonds in the reactants are broken with the energy released w ...
Sec. 10.3 - Midland Park School District
... volumes by using Avogadro’s principle. Recognize the mole relationships shown by a chemical formula. Determine the number of atoms or ions in a mass of a compound. ...
... volumes by using Avogadro’s principle. Recognize the mole relationships shown by a chemical formula. Determine the number of atoms or ions in a mass of a compound. ...
CHEM_1305_Practice_Exam_2
... A) Solid sodium carbonate decomposes to give solid sodium oxide and carbon dioxide gas. B) Sodium carbonate decomposes to form sodium oxide and carbon dioxide. C) Sodium oxide combines with carbon dioxide to form sodium carbonate. D) Sodium oxide is decomposes to give sodium carbonate and carbon dio ...
... A) Solid sodium carbonate decomposes to give solid sodium oxide and carbon dioxide gas. B) Sodium carbonate decomposes to form sodium oxide and carbon dioxide. C) Sodium oxide combines with carbon dioxide to form sodium carbonate. D) Sodium oxide is decomposes to give sodium carbonate and carbon dio ...
Review Material
... Relative Sizes of Ions For the Representative (s-block and p-block) Elements that form positive ions (cations), the radius of the positive ion will always be smaller than the radius of the corresponding atom. This is due primarily to the fact that when these elements form ions the outermost shell (h ...
... Relative Sizes of Ions For the Representative (s-block and p-block) Elements that form positive ions (cations), the radius of the positive ion will always be smaller than the radius of the corresponding atom. This is due primarily to the fact that when these elements form ions the outermost shell (h ...
Mole-Volume Conversion Assignment
... 8. Get two pieces of filter paper. Weigh both together and record the mass: ______________________ g 9. With the pieces of filter paper together, fold them to make a cone (you may want to wet the filter paper). Place the empty beaker under the funnel. Swirl the mixture & pour it into the funnel. Add ...
... 8. Get two pieces of filter paper. Weigh both together and record the mass: ______________________ g 9. With the pieces of filter paper together, fold them to make a cone (you may want to wet the filter paper). Place the empty beaker under the funnel. Swirl the mixture & pour it into the funnel. Add ...
Comparing Free Energies
... DGorxn(T) = DHorxn – T DSorxn at different temperatures if we assume that the values of DSorxn and DHorxn remain constant at all temperatures (which is not necessarily the case, but allows us to make reasonable predictions). To facilitate the calculation of DGorxn at 25 oC and 1 atm for a chemical r ...
... DGorxn(T) = DHorxn – T DSorxn at different temperatures if we assume that the values of DSorxn and DHorxn remain constant at all temperatures (which is not necessarily the case, but allows us to make reasonable predictions). To facilitate the calculation of DGorxn at 25 oC and 1 atm for a chemical r ...
ANSWERS Problem Set 5a – Chemical Reactions
... 8) The reaction below is balanced, but there are only ten molecules of reactants while there are twelve molecules of products. How can this be balanced? C6H12 + 9 O2 6 CO2 + 6 H2O Chemical reactions are not balanced by molecules or compounds, but only by atomic values. The number of atoms that ar ...
... 8) The reaction below is balanced, but there are only ten molecules of reactants while there are twelve molecules of products. How can this be balanced? C6H12 + 9 O2 6 CO2 + 6 H2O Chemical reactions are not balanced by molecules or compounds, but only by atomic values. The number of atoms that ar ...
Unit 2 Chemical Reactions
... Thus it can be noted that the activity series is useful because it indicates the possibility of reaction of a given metal with water, acids, oxygen, sulphur, halogens and compounds of other metals. It also provides a good indication of the relative stability of compounds of any metal. a) one metal d ...
... Thus it can be noted that the activity series is useful because it indicates the possibility of reaction of a given metal with water, acids, oxygen, sulphur, halogens and compounds of other metals. It also provides a good indication of the relative stability of compounds of any metal. a) one metal d ...
CHEMISTRY 102 Spring 2012 Hour Exam III Page 20 1. For the
... After equilibrium is reached, which of the following statements (a-d) regarding these experiments is true? a) In experiment 1, the rate of the forward reaction at equilibrium will be greater than the rate of the reverse reaction at equilibrium. b) Equilibrium cannot be reached in experiment 2 becaus ...
... After equilibrium is reached, which of the following statements (a-d) regarding these experiments is true? a) In experiment 1, the rate of the forward reaction at equilibrium will be greater than the rate of the reverse reaction at equilibrium. b) Equilibrium cannot be reached in experiment 2 becaus ...
Thermo notes Part II
... • Determine whether the entropy value increases or decreases for the following situations Ex. 2) Condensation of water vapor on a window Entropy decreases: a phase change from a disorganized gas to a more organized liquid ∆S (-) ...
... • Determine whether the entropy value increases or decreases for the following situations Ex. 2) Condensation of water vapor on a window Entropy decreases: a phase change from a disorganized gas to a more organized liquid ∆S (-) ...
Chemical Reactions
... • The problem with word equations is they do not actually show the number of atoms or molecules of each substance… formulas would have to be written out for this to happen. (Absent? We looked at examples of these in class) ...
... • The problem with word equations is they do not actually show the number of atoms or molecules of each substance… formulas would have to be written out for this to happen. (Absent? We looked at examples of these in class) ...
Various Types of RXNS
... 5. small chunks of solid sodium are added to water --describe a test to confirm the gaseous product in your reaction 6. hydrobromic acid is added to a solution of potassium hydrogen carbonate --when a gas produced by the reaction is bubbled through limewater, what visible change is expected? 7. aque ...
... 5. small chunks of solid sodium are added to water --describe a test to confirm the gaseous product in your reaction 6. hydrobromic acid is added to a solution of potassium hydrogen carbonate --when a gas produced by the reaction is bubbled through limewater, what visible change is expected? 7. aque ...
Guided notes packet - Science With Horne
... as Gram Formula Weight or Formula Weight.) Mass given on periodic table is in __________________. Because amu and grams are relative, we can say the masses on the periodic table are in _______. To find ______________________________ add all the atomic masses of the elements in the __________________ ...
... as Gram Formula Weight or Formula Weight.) Mass given on periodic table is in __________________. Because amu and grams are relative, we can say the masses on the periodic table are in _______. To find ______________________________ add all the atomic masses of the elements in the __________________ ...
Topic #4 Notes
... When diluting a concentrated acid it is often found that combining water and the acid is a very exothermic process, i.e. one that releases energy. In some cases this energy can be very significant and may even cause the water present to turn into the gaseous state (steam). As the steam leaves the sy ...
... When diluting a concentrated acid it is often found that combining water and the acid is a very exothermic process, i.e. one that releases energy. In some cases this energy can be very significant and may even cause the water present to turn into the gaseous state (steam). As the steam leaves the sy ...
Carbon-12 Stable
... How do we distinguish one example of matter from another? Physical Properties of Matter A Physical Property is something that can be observed or measured without changing the matter’s identity. -They help you identify a substance Examples: Conductivity- ability to transfer heat or electricity State ...
... How do we distinguish one example of matter from another? Physical Properties of Matter A Physical Property is something that can be observed or measured without changing the matter’s identity. -They help you identify a substance Examples: Conductivity- ability to transfer heat or electricity State ...
AP Chemistry Syllabus
... Time Frame: 1 week Topics Covered Naming Compounds (ionic, covalent, acids, simple organic) Mole (Avogadro, molar mass, mole conversions) Percent composition Empirical and molecular formulas Unit 6: Reactions and Stoichiometry Big Ideas: 1, 3 Learning Outcomes: 1.4, 1.18, 3.1, 3.2, 3.3, 3.4, 3.5, 3. ...
... Time Frame: 1 week Topics Covered Naming Compounds (ionic, covalent, acids, simple organic) Mole (Avogadro, molar mass, mole conversions) Percent composition Empirical and molecular formulas Unit 6: Reactions and Stoichiometry Big Ideas: 1, 3 Learning Outcomes: 1.4, 1.18, 3.1, 3.2, 3.3, 3.4, 3.5, 3. ...
AP Chemistry - School Webmasters
... Welcome to AP Chemistry. In order to ensure the best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts some of which you may have forgotten you learned. For those topics you need help with there are a multitude of tremendous chemistry resources a ...
... Welcome to AP Chemistry. In order to ensure the best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts some of which you may have forgotten you learned. For those topics you need help with there are a multitude of tremendous chemistry resources a ...
Stoichiometry

Stoichiometry /ˌstɔɪkiˈɒmɨtri/ is the calculation of relative quantities of reactants and products in chemical reactions.Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of product can be empirically determined, then the amount of the other reactants can also be calculated.As seen in the image to the right, where the balanced equation is:CH4 + 2 O2 → CO2 + 2 H2O.Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. Stoichiometry measures these quantitative relationships, and is used to determine the amount of products/reactants that are produced/needed in a given reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry. In the example above, reaction stoichiometry measures the relationship between the methane and oxygen as they react to form carbon dioxide and water.Because of the well known relationship of moles to atomic weights, the ratios that are arrived at by stoichiometry can be used to determine quantities by weight in a reaction described by a balanced equation. This is called composition stoichiometry.Gas stoichiometry deals with reactions involving gases, where the gases are at a known temperature, pressure, and volume and can be assumed to be ideal gases. For gases, the volume ratio is ideally the same by the ideal gas law, but the mass ratio of a single reaction has to be calculated from the molecular masses of the reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the mass ratio.