L2 Atomic and Nuclear Physics 1
... electric and magnetic forces. 2. Strong interactions responsible for forces between quarks & gluons and nuclear binding. 3. Weak interactions responsible for the instability of all but the least massive fundamental particles. 4. Gravitational interactions responsible for forces between any two objec ...
... electric and magnetic forces. 2. Strong interactions responsible for forces between quarks & gluons and nuclear binding. 3. Weak interactions responsible for the instability of all but the least massive fundamental particles. 4. Gravitational interactions responsible for forces between any two objec ...
Lecture 2
... interaction of electrical charges. Electric and magnetic effects are caused by the relative positions and movements of charged particles of matter. When a charge is stationary (static), it produces electrostatic forces on charged objects, and when it is in motion it produces additional magnetic effe ...
... interaction of electrical charges. Electric and magnetic effects are caused by the relative positions and movements of charged particles of matter. When a charge is stationary (static), it produces electrostatic forces on charged objects, and when it is in motion it produces additional magnetic effe ...
Chem practice sheets CP
... Mass of a proton = _______ amu (atomic mass unit) Mass of a neutron = _______ ...
... Mass of a proton = _______ amu (atomic mass unit) Mass of a neutron = _______ ...
Modern Physics - Tarleton State University
... The probability density for the hydrogen atom for three different electron states. ...
... The probability density for the hydrogen atom for three different electron states. ...
CHAPTER 4: ABUNDANCE AND RADIOACTIVITY OF UNSTABLE
... thorium series. The emission of the heavy a particle practically coincides with the emission of relatively large-energy g rays. Because as in all cases the reaction energy Q is shared by the atoms Y and He according to the two conservation laws, the case of a emission is special. As with a gun shot, ...
... thorium series. The emission of the heavy a particle practically coincides with the emission of relatively large-energy g rays. Because as in all cases the reaction energy Q is shared by the atoms Y and He according to the two conservation laws, the case of a emission is special. As with a gun shot, ...
The Age of the Earth
... protons and two neutrons) and beta decays (neutron changes into proton when the electron leaves). How does alpha decay work? Big nuclei, with 210 or more protons+neutrons, are so large that the shortrange nuclear forces that hold them together are barely able to counterbalance the mutual repulsion o ...
... protons and two neutrons) and beta decays (neutron changes into proton when the electron leaves). How does alpha decay work? Big nuclei, with 210 or more protons+neutrons, are so large that the shortrange nuclear forces that hold them together are barely able to counterbalance the mutual repulsion o ...
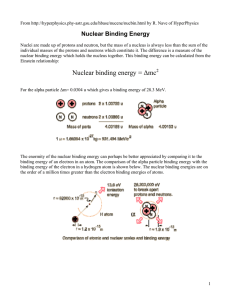
Nuclear binding energy = Δmc2 - University of Toronto Physics
... the order of a million times greater than the electron binding energies of atoms. ...
... the order of a million times greater than the electron binding energies of atoms. ...
Topic 2 - Jensen Chemistry
... Cathode rays have identical properties regardless of the element used to produce them. All elements must contain identically charged electrons. Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons Electrons have so little mass that a ...
... Cathode rays have identical properties regardless of the element used to produce them. All elements must contain identically charged electrons. Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons Electrons have so little mass that a ...
Chemistry Honors Unit 2 Study Guide Atomic Theory Mr. Brown Use
... measured the charge to mass ratio of the electron. He came up with the plum pudding model of the atom in which the electrons were thought to be evenly distributed throughout the atom as raisins are throughout a pudding. Robert Millikan- He measured the charge on an electron in his famous oil drop ex ...
... measured the charge to mass ratio of the electron. He came up with the plum pudding model of the atom in which the electrons were thought to be evenly distributed throughout the atom as raisins are throughout a pudding. Robert Millikan- He measured the charge on an electron in his famous oil drop ex ...
NUCLEAR CHEMISTRY REVIEW SHEET
... a. It increases by four b. It decreases by one c. It decreases by four d. It remains the same _____ 10. When an atom undergoes radioactive decay by emitting an alpha particle, what change occurs to the atomic number of that atom? a. It increases by one b. It decreases by one c. It increases by two d ...
... a. It increases by four b. It decreases by one c. It decreases by four d. It remains the same _____ 10. When an atom undergoes radioactive decay by emitting an alpha particle, what change occurs to the atomic number of that atom? a. It increases by one b. It decreases by one c. It increases by two d ...
Revision of Atomic Structure and Nuclide Notations Nuclide
... The atoms of most elements have isotopes. Some of these isotopes have nuclei which are unstable and give out different types of particles or rays. This release (emission) of particles and rays is what we call radiation and helps to make the atom more stable. Radioactivity is all around us. We are co ...
... The atoms of most elements have isotopes. Some of these isotopes have nuclei which are unstable and give out different types of particles or rays. This release (emission) of particles and rays is what we call radiation and helps to make the atom more stable. Radioactivity is all around us. We are co ...
Chp 7.1 Atomic Theory and Radioactive Decay
... • Radioactivity results from having an unstable nucleus. • When these nuclei lose energy and break apart, decay occurs. • Radioactive decay releases energy from the nucleus as radiation. • Radioactive atoms release energy until they become stable, often as different atoms. • An element may have only ...
... • Radioactivity results from having an unstable nucleus. • When these nuclei lose energy and break apart, decay occurs. • Radioactive decay releases energy from the nucleus as radiation. • Radioactive atoms release energy until they become stable, often as different atoms. • An element may have only ...
10th Grade Chemistry X (TJ) GRADE(S)/LEVELS SUBJECT Power
... Solutions are mixtures in which particles of one substance are evenly distributed through another substance. Liquids are limited in the amount of dissolved solid or gas that they can contain. Aqueous solutions can be described by relative quantities of the dissolved substances and acidity or alkalin ...
... Solutions are mixtures in which particles of one substance are evenly distributed through another substance. Liquids are limited in the amount of dissolved solid or gas that they can contain. Aqueous solutions can be described by relative quantities of the dissolved substances and acidity or alkalin ...
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.