Atoms and Periodic Table Unit Name
... heat and electricity. They also have luster and a high density 27 - Metals are considered this if they can be made into wire. 29 - There are this many known quarks? 30 - The attraction that holds atoms close to each other 32 - Group of nitrogenous organic compounds that are essential parts of ...
... heat and electricity. They also have luster and a high density 27 - Metals are considered this if they can be made into wire. 29 - There are this many known quarks? 30 - The attraction that holds atoms close to each other 32 - Group of nitrogenous organic compounds that are essential parts of ...
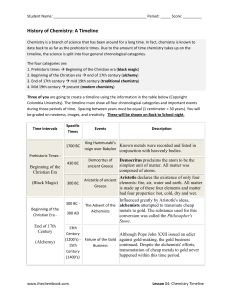
Word - The Chemistry Book
... Ernest Rutherford sent a radioactive source through a magnetic field. Some of the radioactivity was deflected to the positive plate; some of it was deflected to the negative plate; and the rest went through the magnetic field without deflection. Thus, there were three types of radioactivity: alpha p ...
... Ernest Rutherford sent a radioactive source through a magnetic field. Some of the radioactivity was deflected to the positive plate; some of it was deflected to the negative plate; and the rest went through the magnetic field without deflection. Thus, there were three types of radioactivity: alpha p ...
Name
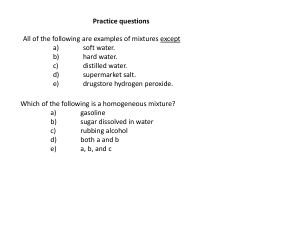
... 2. What are the elements in the chemical formula of caffeine C8H10N4O2? _______________________________________________________________________________________ 3. How many atoms are in sulfuric acid H2SO4? How many atoms of Sulfur are in the formula? _________________________________________________ ...
... 2. What are the elements in the chemical formula of caffeine C8H10N4O2? _______________________________________________________________________________________ 3. How many atoms are in sulfuric acid H2SO4? How many atoms of Sulfur are in the formula? _________________________________________________ ...
Chemistry PowerPoint
... Which atomic particle is correctly matched with its location? a. Proton; orbits the nucleus b. Neutron; orbits the nucleus c. Electron; in the nucleus d. Proton; in the nucleus ...
... Which atomic particle is correctly matched with its location? a. Proton; orbits the nucleus b. Neutron; orbits the nucleus c. Electron; in the nucleus d. Proton; in the nucleus ...
Monday, Feb. 7, 2005
... • If the nuclear force is long-ranged and is independent of the presence of other nucleons, BE per nucleon will increase linearly with A – This is because long-range forces do not saturate – Since any single particle can interact with as many other particle as are available Binding becomes tighter ...
... • If the nuclear force is long-ranged and is independent of the presence of other nucleons, BE per nucleon will increase linearly with A – This is because long-range forces do not saturate – Since any single particle can interact with as many other particle as are available Binding becomes tighter ...
AP Chemistry Summer Assignment - Belle Vernon Area School District
... 2. You need to master the formulas, charges, and names of the common ions. On the first week of the school year, you will be given a quiz on these ions. You will be asked to: • write the names of these ions when given the formula and charge • write the formula and charge when given the names I have ...
... 2. You need to master the formulas, charges, and names of the common ions. On the first week of the school year, you will be given a quiz on these ions. You will be asked to: • write the names of these ions when given the formula and charge • write the formula and charge when given the names I have ...
What are the four fundamental forces of nature?
... In the 1950s, physicists built particle accelerators to explore the structure of the nucleus. When they crashed atoms together at high speeds, they found the pions predicted by Yukawa. They also found that protons and neutrons were made of smaller particles called quarks. So, the strong force held ...
... In the 1950s, physicists built particle accelerators to explore the structure of the nucleus. When they crashed atoms together at high speeds, they found the pions predicted by Yukawa. They also found that protons and neutrons were made of smaller particles called quarks. So, the strong force held ...
Neutrons and Protons
... 5.06x10-29 kg of mass released as energy when protons & neutrons combined to form Helium nucleus. This is the ‘binding’ energy of the nucleus. ...
... 5.06x10-29 kg of mass released as energy when protons & neutrons combined to form Helium nucleus. This is the ‘binding’ energy of the nucleus. ...
File
... C) It has a charge of -13 and is surrounded by a total of 10 electrons. D) It has a charge of -13 and is surrounded by a total of 13 electrons. 4. The mass of an electron is approximately equal to of the mass of A) a positron B) a proton C) an alpha particle D) a beta particle 5. Compared to the ent ...
... C) It has a charge of -13 and is surrounded by a total of 10 electrons. D) It has a charge of -13 and is surrounded by a total of 13 electrons. 4. The mass of an electron is approximately equal to of the mass of A) a positron B) a proton C) an alpha particle D) a beta particle 5. Compared to the ent ...
Life in the Higgs condensate, where electrons have mass
... The particle recently discovered by the CMS and ATLAS collaborations at CERN is almost certainly a Higgs boson, fulfilling a quest that can be traced back to three seminal high-energy papers of 1964, but which is intimately connected to ideas in other areas of physics that go back much further. In 1 ...
... The particle recently discovered by the CMS and ATLAS collaborations at CERN is almost certainly a Higgs boson, fulfilling a quest that can be traced back to three seminal high-energy papers of 1964, but which is intimately connected to ideas in other areas of physics that go back much further. In 1 ...
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.