Nuclear Final Exam
... Answer question 4, it is a middle complexity type problem, it is not really that hard once you understand the statistical view of the problem, I expect to see a derivation of the total probability here. (10 pt problem) 4. Lets say that you’re a nuclear physicsts who is working on determining the nu ...
... Answer question 4, it is a middle complexity type problem, it is not really that hard once you understand the statistical view of the problem, I expect to see a derivation of the total probability here. (10 pt problem) 4. Lets say that you’re a nuclear physicsts who is working on determining the nu ...
Materials Science
... Electrons in Atoms Electrons move not in circular orbits, but in 'fuzzy‘ orbits. Actually, we cannot tell how it moves, but only can say what is the probability of finding it at some distance from the nucleus. Only certain “orbits” or shells of electron probability densities are allowed. The shell ...
... Electrons in Atoms Electrons move not in circular orbits, but in 'fuzzy‘ orbits. Actually, we cannot tell how it moves, but only can say what is the probability of finding it at some distance from the nucleus. Only certain “orbits” or shells of electron probability densities are allowed. The shell ...
Gateway Chemistry Review (Answer Key) Structure and Properties
... o Faster moving particles have greater average kinetic energy. o The more kinetic energy particles have, the greater the temperature of the object or substance. ...
... o Faster moving particles have greater average kinetic energy. o The more kinetic energy particles have, the greater the temperature of the object or substance. ...
Please look over the following review questions
... course for the information. Do not submit these to your instructor. ...
... course for the information. Do not submit these to your instructor. ...
4.2 The Quantum Model of the Atom Vocab Electromagnetic
... - A state in which an atom has more energy than it does at its ground state. Emission-Line Spectrum - A diagram or graph that indicates the degree to which a substance emits radiant energy with respect to wavelength. Continuous Spectrum The uninterrupted broad band of all colors [wavelengths] emitte ...
... - A state in which an atom has more energy than it does at its ground state. Emission-Line Spectrum - A diagram or graph that indicates the degree to which a substance emits radiant energy with respect to wavelength. Continuous Spectrum The uninterrupted broad band of all colors [wavelengths] emitte ...
Part a
... Chemical energy—stored in bonds of chemical substances Electrical energy—results from movement of charged particles Mechanical energy—directly involved in moving matter Radiant or electromagnetic energy—exhibits wavelike properties (i.e., visible light, ultraviolet light, and X-rays) ...
... Chemical energy—stored in bonds of chemical substances Electrical energy—results from movement of charged particles Mechanical energy—directly involved in moving matter Radiant or electromagnetic energy—exhibits wavelike properties (i.e., visible light, ultraviolet light, and X-rays) ...
The Strong interaction or the mystery of the nucleus - Pierre
... Hadrons are colourless objects ...
... Hadrons are colourless objects ...
Preview Sample 1
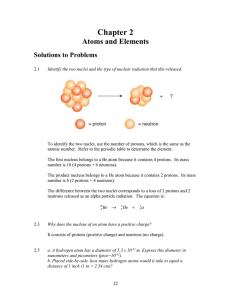
... The product nucleus belongs to a He atom because it contains 2 protons. Its mass number is 6 (2 protons + 4 neutrons). The difference between the two nuclei corresponds to a loss of 2 protons and 2 neutrons released as an alpha particle radiation. The equation is: ...
... The product nucleus belongs to a He atom because it contains 2 protons. Its mass number is 6 (2 protons + 4 neutrons). The difference between the two nuclei corresponds to a loss of 2 protons and 2 neutrons released as an alpha particle radiation. The equation is: ...
chemia simr01 en - Leszek Niedzicki
... abundant ones we have: ionic (electrostatic interactions between two ions), covalent (bond is formed through equal electron input from both atoms which are of similar electronegativity) and coordination (all electrons in a bond come from only one atom) ones. • Energy of chemical bond between given a ...
... abundant ones we have: ionic (electrostatic interactions between two ions), covalent (bond is formed through equal electron input from both atoms which are of similar electronegativity) and coordination (all electrons in a bond come from only one atom) ones. • Energy of chemical bond between given a ...
Energy Level diagram for a spin-1/2 nucleus as a function of
... The z-component of the nuclear angular momentum is related to the z-component of the nuclear spin. Recall in quantum mechanics that a total angular momentum of L has 2(L+1) components. The same is true for nuclear angular momentum and hence spin. (this gives rise to the different orbital structures) ...
... The z-component of the nuclear angular momentum is related to the z-component of the nuclear spin. Recall in quantum mechanics that a total angular momentum of L has 2(L+1) components. The same is true for nuclear angular momentum and hence spin. (this gives rise to the different orbital structures) ...
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.