ap physics ii exam -2015
... 1) Objects and systems have properties such as mass and charge. Systems may have internal structures. 2) Fields existing in space can be used to explain interactions. 3) The interactions of an object with other objects can be described by forces. 4) Interactions between systems can result in changes ...
... 1) Objects and systems have properties such as mass and charge. Systems may have internal structures. 2) Fields existing in space can be used to explain interactions. 3) The interactions of an object with other objects can be described by forces. 4) Interactions between systems can result in changes ...
Worksheet 4 - Periodic Trends A number of physical and chemical
... These properties all involve the outer shell (valence) electrons as well as the inner shell (shielding) electrons. Electrons are held in the atom by their electrostatic attraction to the positively charged protons, the nuclear charge, Z. However, not all electrons in an atom experience the same nucl ...
... These properties all involve the outer shell (valence) electrons as well as the inner shell (shielding) electrons. Electrons are held in the atom by their electrostatic attraction to the positively charged protons, the nuclear charge, Z. However, not all electrons in an atom experience the same nucl ...
Average Atomic Mass
... 62. Calculate the molar mass of magnesium phosphate. 63. How many moles are in 7.23 grams of strontium oxide? 64. How many moles are in 3.02 x 1023 atoms of zinc? 65. How many grams are in 7.2 x 1046 molecules of copper (II) sulfate? 66. How many grams are in 1.00 moles of sodium oxalate? 67. How ma ...
... 62. Calculate the molar mass of magnesium phosphate. 63. How many moles are in 7.23 grams of strontium oxide? 64. How many moles are in 3.02 x 1023 atoms of zinc? 65. How many grams are in 7.2 x 1046 molecules of copper (II) sulfate? 66. How many grams are in 1.00 moles of sodium oxalate? 67. How ma ...
Chemistry I - Net Start Class
... 2. Which of the following is a physical change? Water changes to steam. A mixture of baking soda and vinegar give off a gas. Heated moth balls turn into a liquid. A powder mixed with water makes lemonade. 3. A solid, silver-colored metallic element is combined with a light green, gaseous element, pr ...
... 2. Which of the following is a physical change? Water changes to steam. A mixture of baking soda and vinegar give off a gas. Heated moth balls turn into a liquid. A powder mixed with water makes lemonade. 3. A solid, silver-colored metallic element is combined with a light green, gaseous element, pr ...
投影片 - 中正大學化生系
... 3. The arrangement of the elements in groups of elements in the order of their atomic weights corresponds to their so-called valencies, as well as, to some extent, to their distinctive chemical properties; as is apparent among other series in that of Li, Be, B, C, N, O, and F. 4. The magnitude of th ...
... 3. The arrangement of the elements in groups of elements in the order of their atomic weights corresponds to their so-called valencies, as well as, to some extent, to their distinctive chemical properties; as is apparent among other series in that of Li, Be, B, C, N, O, and F. 4. The magnitude of th ...
The Atomic Theory
... different elements are different. 3. Atoms of an element are not changed into different ...
... different elements are different. 3. Atoms of an element are not changed into different ...
The Structure of the Atom
... A. J.J. Thomson – 1890s B. Robert Millikan - 1909 C. Rutherford - 1911 1. Fired alpha particles at thin metal sheet. 2. Expected them to go straight through, but some deflected. 3. This led to nuclear model. ...
... A. J.J. Thomson – 1890s B. Robert Millikan - 1909 C. Rutherford - 1911 1. Fired alpha particles at thin metal sheet. 2. Expected them to go straight through, but some deflected. 3. This led to nuclear model. ...
Name - Net Start Class
... c. Solution - a homogeneous mixture that consists of a solvent and at least one solute. 8. What process would best be used to separate out a salt, sand, and iron filling mixture? Remove iron fillings with a magnet; add water to the remaining salt and sand mixture; pour the mixture through filter pap ...
... c. Solution - a homogeneous mixture that consists of a solvent and at least one solute. 8. What process would best be used to separate out a salt, sand, and iron filling mixture? Remove iron fillings with a magnet; add water to the remaining salt and sand mixture; pour the mixture through filter pap ...
Lecture 1
... Two equally charged particles are held 2.5X 10-3 m apart and then released from rest. The initial acceleration of the first particle is observed to be 5.0 m/s2 and that of the second to be 11.0 m/s2. The mass of the first particle is 6.3X10-7 kg. What is the mass of the second particle? [2.86e-07] k ...
... Two equally charged particles are held 2.5X 10-3 m apart and then released from rest. The initial acceleration of the first particle is observed to be 5.0 m/s2 and that of the second to be 11.0 m/s2. The mass of the first particle is 6.3X10-7 kg. What is the mass of the second particle? [2.86e-07] k ...
File
... For Tertiary Compounds (two or more elements): First, write the name of the positive ion Then, write the name of the polyatomic ion, or if the negative ion is a single element, change the ending to –ide Examples: Mg+2 and NO2- become magnesium nitrite NH4+ and Cl- become ammonium chloride ...
... For Tertiary Compounds (two or more elements): First, write the name of the positive ion Then, write the name of the polyatomic ion, or if the negative ion is a single element, change the ending to –ide Examples: Mg+2 and NO2- become magnesium nitrite NH4+ and Cl- become ammonium chloride ...
Exam 3 Review - Iowa State University
... 8. Which of the following has the smallest ionization energy. a. Mg b. Se c. Ba d. Po 9. Which has the largest 2nd Ionization energy between K and Ca? a. K b. Ca c. Both K and Ca have the same second Ionization energy d. It’s impossible to tell 10. In terms of electronegativity, determine whether th ...
... 8. Which of the following has the smallest ionization energy. a. Mg b. Se c. Ba d. Po 9. Which has the largest 2nd Ionization energy between K and Ca? a. K b. Ca c. Both K and Ca have the same second Ionization energy d. It’s impossible to tell 10. In terms of electronegativity, determine whether th ...
Physics 9 Fall 2010 - faculty.ucmerced.edu
... We could try to figure out the components of the electric field, and integrate it over the surface of the cube, but there’s a much easier and more clever way. Suppose that we imagine stacking other boxes around the cube, keeping the charge at the center, as seen in the figure to the right. The net f ...
... We could try to figure out the components of the electric field, and integrate it over the surface of the cube, but there’s a much easier and more clever way. Suppose that we imagine stacking other boxes around the cube, keeping the charge at the center, as seen in the figure to the right. The net f ...
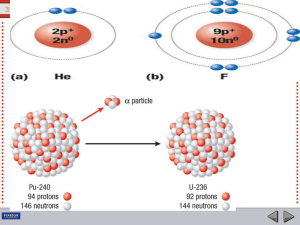
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.