Coherence and Indistinguishability of Single Electrons Emitted by
... The production of indistinguishable partners is challenging and their generation by independent sources has been only recently achieved in optics (8). In one dimensional quantum conductors, a continuous stream of indistinguishable electrons can be produced by applying a ...
... The production of indistinguishable partners is challenging and their generation by independent sources has been only recently achieved in optics (8). In one dimensional quantum conductors, a continuous stream of indistinguishable electrons can be produced by applying a ...
printer-friendly version of benchmark
... Thomson continued experimenting with the cathode ray tube in order to determine some of the characteristics of the electron. Because the ray of electrons bent towards the positive electrode of the cathode ray tube, Thomson knew that the electron was negatively charged. He was unable to determine the ...
... Thomson continued experimenting with the cathode ray tube in order to determine some of the characteristics of the electron. Because the ray of electrons bent towards the positive electrode of the cathode ray tube, Thomson knew that the electron was negatively charged. He was unable to determine the ...
Electric Charge and Static Electricity
... Atoms have equal numbers of protons (positive) and electrons (negative) therefore, atoms are neutral, with no charge. An atom that loses electrons will have more protons than electrons = positive charge. An atom that gains electrons will have more electrons than protons = negative charge. ...
... Atoms have equal numbers of protons (positive) and electrons (negative) therefore, atoms are neutral, with no charge. An atom that loses electrons will have more protons than electrons = positive charge. An atom that gains electrons will have more electrons than protons = negative charge. ...
contents - Jordan University of Science and Technology
... J K J and K Q K , J and Jf are the total angular momentum for the initial and final states of the system respectively. ...
... J K J and K Q K , J and Jf are the total angular momentum for the initial and final states of the system respectively. ...
Lower-Hybrid Waves
... simple calculation below confirms this argument. To estimate the ion temperature we utilize the fact that the ratio of ion and electron drifts is given by V T Di ...
... simple calculation below confirms this argument. To estimate the ion temperature we utilize the fact that the ratio of ion and electron drifts is given by V T Di ...
Quantum Numbers
... • No exact solution to the Schrödinger equation is known for systems with two or more electrons • We make the approximation that the electrons in a polyelectronic atom are in atomic orbitals that resemble those found within the hydrogen atom (for which the exact solutions are known) • N.B. The 1s, 2 ...
... • No exact solution to the Schrödinger equation is known for systems with two or more electrons • We make the approximation that the electrons in a polyelectronic atom are in atomic orbitals that resemble those found within the hydrogen atom (for which the exact solutions are known) • N.B. The 1s, 2 ...
Rdg: Electron Configuration
... I. Principle Quantum Number (n) and Sublevels The number of sublevels that an energy level can contain is equal to the principle quantum number of that level. So, for example, the second energy level would have two sublevels, and the third energy level would have three sublevels. The first sublevel ...
... I. Principle Quantum Number (n) and Sublevels The number of sublevels that an energy level can contain is equal to the principle quantum number of that level. So, for example, the second energy level would have two sublevels, and the third energy level would have three sublevels. The first sublevel ...
EOC_chapter28
... electromagnetic waves. (c) If it were alone in the Universe, at what rate would its temperature be changing? (d) Assume that Wien’s law describes the sphere. Find the wavelength λmax of electromagnetic radiation it emits most strongly. Although it emits a spectrum of waves having all different wavel ...
... electromagnetic waves. (c) If it were alone in the Universe, at what rate would its temperature be changing? (d) Assume that Wien’s law describes the sphere. Find the wavelength λmax of electromagnetic radiation it emits most strongly. Although it emits a spectrum of waves having all different wavel ...
Inertial Mass and Gravitational Mass - What They Are and
... objects. A neutral object is one which contains equal amounts of negative charges and positive charges. Each electron and proton in the neutral object behaves as the isolated electron discussed above[1]. The only difference is that, whereas the magnetic field of the isolated electron can be detected ...
... objects. A neutral object is one which contains equal amounts of negative charges and positive charges. Each electron and proton in the neutral object behaves as the isolated electron discussed above[1]. The only difference is that, whereas the magnetic field of the isolated electron can be detected ...
2.4 Particle interactions
... The strong nuclear force acts between all particles containing quarks, which are constituents of particles (neutrons and protons) in the nucleus. It acts over a very short range. The exchange particle for the strong nuclear force is the pion. ...
... The strong nuclear force acts between all particles containing quarks, which are constituents of particles (neutrons and protons) in the nucleus. It acts over a very short range. The exchange particle for the strong nuclear force is the pion. ...
Module 2 ATOMIC STRUCTURE
... by a metal, the total energy of the photon, hν is given to a single electron within the metal. If this energy is sufficiently large, the electron may penetrate the potential barrier at the surface of the metal and still retain some energy as kinetic energy. The kinetic energy retained by the electro ...
... by a metal, the total energy of the photon, hν is given to a single electron within the metal. If this energy is sufficiently large, the electron may penetrate the potential barrier at the surface of the metal and still retain some energy as kinetic energy. The kinetic energy retained by the electro ...
Chapter 4 Assignment Answers
... b. Thomson observed the same cathode rays with all of the different metals that he used. 39. Two electrons should repel each other. 40. The mass of a neutron is equal to the mass of a proton: 1 amu. However a proton is (+) charged and a neutron is neutral. 41. When an atom loses electrons, there are ...
... b. Thomson observed the same cathode rays with all of the different metals that he used. 39. Two electrons should repel each other. 40. The mass of a neutron is equal to the mass of a proton: 1 amu. However a proton is (+) charged and a neutron is neutral. 41. When an atom loses electrons, there are ...
PPT
... The problem: If electrons are magnetized, they merely drift, no matter how hard you try to accelerate them. ...
... The problem: If electrons are magnetized, they merely drift, no matter how hard you try to accelerate them. ...
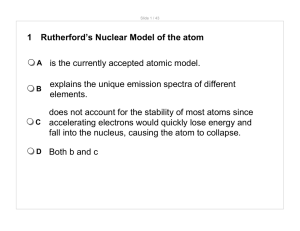
1 Rutherford`s Nuclear Model of the atom A is the currently accepted
... The lowest orbital energy is reached when the number of electrons with the same spin is maximized. This statement describes __________. A ...
... The lowest orbital energy is reached when the number of electrons with the same spin is maximized. This statement describes __________. A ...
One-dimensional electron transport in
... electron–electron interaction could dominate the behaviour of electrons in a confined environment. Experiments on wider devices have revealed the stages of formation of the incipient lattice. As the ration of interaction to confinement is increased, electrons start to move sideways and then form a ‘ ...
... electron–electron interaction could dominate the behaviour of electrons in a confined environment. Experiments on wider devices have revealed the stages of formation of the incipient lattice. As the ration of interaction to confinement is increased, electrons start to move sideways and then form a ‘ ...
File
... Charges on ions • When atoms form ions they aim to attain electron shells that are either completely full or completely empty. • If we know the electron configuration of an atom we can usually work out how many electrons it must lose or gain to achieve a noble gas configuration. • This will tell us ...
... Charges on ions • When atoms form ions they aim to attain electron shells that are either completely full or completely empty. • If we know the electron configuration of an atom we can usually work out how many electrons it must lose or gain to achieve a noble gas configuration. • This will tell us ...
Non-destructive, Absolute Mass Determination of Sub
... resembling the ring electrode of a Paul-trap. This open design has been chosen in order to obtain a large solid angle for light detection and also to access the trap volume through several ports with additional tools such as the particle source, the laser beam and the electron gun. The potential ( 0 ...
... resembling the ring electrode of a Paul-trap. This open design has been chosen in order to obtain a large solid angle for light detection and also to access the trap volume through several ports with additional tools such as the particle source, the laser beam and the electron gun. The potential ( 0 ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.