Radioactivity - MrSimonPorter
... physicist called Niels Bohr realised that the secret of atomic structure lay in its discreteness, that energy could only be absorbed or emitted at certain values. ...
... physicist called Niels Bohr realised that the secret of atomic structure lay in its discreteness, that energy could only be absorbed or emitted at certain values. ...
Modern physics
... to the frequency of the light, but does not depend on its intensity Compton effect was of great historical importance because it confirmed that photons are real particles with momentum as well as energy. Collisions between the energetic quanta of radiation and electrons obey relativistic energy and ...
... to the frequency of the light, but does not depend on its intensity Compton effect was of great historical importance because it confirmed that photons are real particles with momentum as well as energy. Collisions between the energetic quanta of radiation and electrons obey relativistic energy and ...
Fulltext PDF
... 4. How Particles Collide According to Quantum Field Theory So far, we have talked about electrons and quarks. They are spin-1/2 fermions. We also have photons – particles of light, which are spin-1 bosons. (The Higgs particle is a spin-0 boson). The paradigm of particle physics is that these particl ...
... 4. How Particles Collide According to Quantum Field Theory So far, we have talked about electrons and quarks. They are spin-1/2 fermions. We also have photons – particles of light, which are spin-1 bosons. (The Higgs particle is a spin-0 boson). The paradigm of particle physics is that these particl ...
Lecture note--Atomic Models
... Atomic Model of the Atom • Electrons reside in “orbitals” surrounding the nucleus of the atom. • There are four types of electron orbitals: s, p, d, and f. An orbital describes the probability of finding the electron at a certain location around the nucleus. A picture of the shapes of the different ...
... Atomic Model of the Atom • Electrons reside in “orbitals” surrounding the nucleus of the atom. • There are four types of electron orbitals: s, p, d, and f. An orbital describes the probability of finding the electron at a certain location around the nucleus. A picture of the shapes of the different ...
Electrostatics
... scientist coined the term from the Greek root “elektron” meaning amber. Amber was the material that ancient Greek philosophers had noticed would mysteriously attract small particles after it had been rubbed with fur. ...
... scientist coined the term from the Greek root “elektron” meaning amber. Amber was the material that ancient Greek philosophers had noticed would mysteriously attract small particles after it had been rubbed with fur. ...
Electricity Powerpoint
... electrical force exerted on an object is related to the following: 1. Distance from the charged object 2. Strength of the charge on the charged object ...
... electrical force exerted on an object is related to the following: 1. Distance from the charged object 2. Strength of the charge on the charged object ...
What 3 ways can things become charged?
... electrical force exerted on an object is related to the following: 1. Distance from the charged object 2. Strength of the charge on the charged object ...
... electrical force exerted on an object is related to the following: 1. Distance from the charged object 2. Strength of the charge on the charged object ...
semiconductor_overview - Lane Department of Computer
... and hole (h+) pair • h+ is simply a missing electron, which leaves an excess positive charge (due to an extra proton) • Recombination – if an e- and an h+ come in contact, they annihilate each other • Electrons and holes are called “carriers” because they are charged particles – when they move, they ...
... and hole (h+) pair • h+ is simply a missing electron, which leaves an excess positive charge (due to an extra proton) • Recombination – if an e- and an h+ come in contact, they annihilate each other • Electrons and holes are called “carriers” because they are charged particles – when they move, they ...
Energy Band Diagrams - West Virginia University
... and hole (h+) pair • h+ is simply a missing electron, which leaves an excess positive charge (due to an extra proton) • Recombination – if an e- and an h+ come in contact, they annihilate each other • Electrons and holes are called “carriers” because they are charged particles – when they move, they ...
... and hole (h+) pair • h+ is simply a missing electron, which leaves an excess positive charge (due to an extra proton) • Recombination – if an e- and an h+ come in contact, they annihilate each other • Electrons and holes are called “carriers” because they are charged particles – when they move, they ...
2.1 Optical and Thermal Properties of Gold Nanoparticles
... solution already exists. Danish physicist Ludvik Lorenz first published this in 1890, however only in Danish. Later, Gustav Mie "rediscovered" it in 1908, wherefore it is generally known as Mie-Theory. The theory is valid for all nanoparticle sizes and optical wavelengths in contrast to Rayleigh’s s ...
... solution already exists. Danish physicist Ludvik Lorenz first published this in 1890, however only in Danish. Later, Gustav Mie "rediscovered" it in 1908, wherefore it is generally known as Mie-Theory. The theory is valid for all nanoparticle sizes and optical wavelengths in contrast to Rayleigh’s s ...
How have advances in particle accelerator technology helped the
... Physicists have long needed a tool that allows them to create and observe fundamental particles that all matter consists of. It had been long expressed by early particle physicists that they require a source of particles of higher energies then those produced in radio-active decay for the study of t ...
... Physicists have long needed a tool that allows them to create and observe fundamental particles that all matter consists of. It had been long expressed by early particle physicists that they require a source of particles of higher energies then those produced in radio-active decay for the study of t ...
electron orbits atomic spectra the Bohr atom
... More specifically, it is not difficult to show that the angular momentum is quantized in units of h/2, which we call ħ. L = n h / 2 = n ħ . ...
... More specifically, it is not difficult to show that the angular momentum is quantized in units of h/2, which we call ħ. L = n h / 2 = n ħ . ...
Modern Physics
... verified?” If matter has a wave-like nature, it should exhibit interference in a manner completely analogous to the interference of light. Thus, when passing through a regular array of slits, or reflecting from a regular array of atoms, an interference pattern should form. In 1927 Clinton Davisson a ...
... verified?” If matter has a wave-like nature, it should exhibit interference in a manner completely analogous to the interference of light. Thus, when passing through a regular array of slits, or reflecting from a regular array of atoms, an interference pattern should form. In 1927 Clinton Davisson a ...
Notes - GRITLab
... Vocabulary Ion when an electron is removed from an atom (normal atoms have same number of electrons and protons) Insulators charge can be rubbed on or off their surfaces, but it tends to stick there and will not move easily through the materials (examples: rubber, glass, plastic) Conductors el ...
... Vocabulary Ion when an electron is removed from an atom (normal atoms have same number of electrons and protons) Insulators charge can be rubbed on or off their surfaces, but it tends to stick there and will not move easily through the materials (examples: rubber, glass, plastic) Conductors el ...
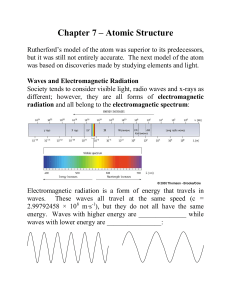
Chapter 2 – Atoms and Elements - U of L Class Index
... either heat or electromagnetic radiation. As such, the electron should constantly be losing energy and therefore slowing down. The negatively charged electron is attracted to the positively charged nucleus so, as it slows, the electron should spiral in toward the nucleus. This does not happen! Why n ...
... either heat or electromagnetic radiation. As such, the electron should constantly be losing energy and therefore slowing down. The negatively charged electron is attracted to the positively charged nucleus so, as it slows, the electron should spiral in toward the nucleus. This does not happen! Why n ...
Answers
... Intrinsic Randomness: Individual results are fundamentally random The spread on the screen is predictable, but where an individual object will land is not. Uncertainty: There are theoretical limits to what can be known. There is a precise mathematical limit to our knowledge of the position and m ...
... Intrinsic Randomness: Individual results are fundamentally random The spread on the screen is predictable, but where an individual object will land is not. Uncertainty: There are theoretical limits to what can be known. There is a precise mathematical limit to our knowledge of the position and m ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.