Atomic Notes
... • An element is a substance made up of only one type of atom. • Atoms can react with other atoms to form compounds. A compound is made up of two or more different types of atoms. • Both before and after a chemical reaction, the number of atoms and the total mass stay the same. Atoms and their subato ...
... • An element is a substance made up of only one type of atom. • Atoms can react with other atoms to form compounds. A compound is made up of two or more different types of atoms. • Both before and after a chemical reaction, the number of atoms and the total mass stay the same. Atoms and their subato ...
7 Periodic Properties of the Elements
... affinity, a gas phase single atom property, of F is less negative than that of Cl, the tendency of F to hold its own electrons (high ionization energy) coupled with a relatively large exothermic electron affinity makes it extremely susceptible to reduction and chemical bond formation. Cl is unreacti ...
... affinity, a gas phase single atom property, of F is less negative than that of Cl, the tendency of F to hold its own electrons (high ionization energy) coupled with a relatively large exothermic electron affinity makes it extremely susceptible to reduction and chemical bond formation. Cl is unreacti ...
Effect of the Spin-Spin Interaction on the Coulomb`s Law
... where D is a coupling constant, m is the mass of an electron, R is the distance between the two electrons, ρo is the massive density of the interacting field, DR/c2 is the “massless density” of the interacting field, ωq = cq is the classical oscillation frequency of the interacting field, qo is the ...
... where D is a coupling constant, m is the mass of an electron, R is the distance between the two electrons, ρo is the massive density of the interacting field, DR/c2 is the “massless density” of the interacting field, ωq = cq is the classical oscillation frequency of the interacting field, qo is the ...
Atomic Electron Configurations and Chapter 8 Chemical Periodicity
... Chapter 8 Atomic Electron Configurations and Chemical Periodicity ...
... Chapter 8 Atomic Electron Configurations and Chemical Periodicity ...
1 Proton and Electron Mass Determination S. Reucroft* and E. G. H.
... We propose simple models to describe the electron and the proton. The electron is pointlike (radius ~ 0) and its mass comes from the combination of electrostatic and gravitation self-energies. The proton model is an atom-like structure with two positively charged electrons in orbit around the third ...
... We propose simple models to describe the electron and the proton. The electron is pointlike (radius ~ 0) and its mass comes from the combination of electrostatic and gravitation self-energies. The proton model is an atom-like structure with two positively charged electrons in orbit around the third ...
Atomic Structure
... blow up). Some radial solutions blow up at r = 0 or r = ∞, and so must be discarded. As in the harmonic oscillator problem, the radial solutions R(r) turn out to be an exponential function ear, multiplied by a polynomial in r. The angular solutions () are polynomials containing powers of sin and ...
... blow up). Some radial solutions blow up at r = 0 or r = ∞, and so must be discarded. As in the harmonic oscillator problem, the radial solutions R(r) turn out to be an exponential function ear, multiplied by a polynomial in r. The angular solutions () are polynomials containing powers of sin and ...
The regularities of the Rydberg energy levels of many
... experimental results within only several percent of 1 cm-1. In addition, there are other methods to calculate the quantum defect number, including the formula presented by Hicks[17], the dynamical improvement of quantum defects by Ganesh Vaidyanathan, et al.[18], the core polarization qualification ...
... experimental results within only several percent of 1 cm-1. In addition, there are other methods to calculate the quantum defect number, including the formula presented by Hicks[17], the dynamical improvement of quantum defects by Ganesh Vaidyanathan, et al.[18], the core polarization qualification ...
study guide: atomic theory quest study guide: atomic
... Describe the structure of an atom using the terms protons, neutrons, electrons, shells, and nucleus Define “subatomic particle” and give the charge and relative mass of the subatomic particles Define atomic number and atomic mass Describe quarks and leptons in terms of what they are and what subatom ...
... Describe the structure of an atom using the terms protons, neutrons, electrons, shells, and nucleus Define “subatomic particle” and give the charge and relative mass of the subatomic particles Define atomic number and atomic mass Describe quarks and leptons in terms of what they are and what subatom ...
ERC-focus (English)
... The up and down quarks and the leptons, the electron and the up to now not mentioned electron-neutrino, constitute a family of elementary particles from which the known matter in the universe is built. The neutrino plays a role in the so-called weak decay of particles through the exchange of yet ano ...
... The up and down quarks and the leptons, the electron and the up to now not mentioned electron-neutrino, constitute a family of elementary particles from which the known matter in the universe is built. The neutrino plays a role in the so-called weak decay of particles through the exchange of yet ano ...
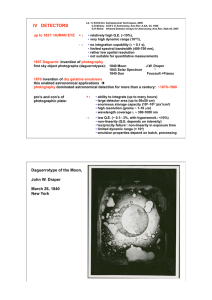
IV DETECTORS
... ‘BIB’ (blocked impurity band) detectors use heavily doped Si which would normally lead to high dark current; to suppress this there is an extra blocking layer of pure Si ...
... ‘BIB’ (blocked impurity band) detectors use heavily doped Si which would normally lead to high dark current; to suppress this there is an extra blocking layer of pure Si ...
Energy Spectra of Auto-Ionizing Electrons in Oxygen
... (1) If neither of the states is an S state, then the autoionizing transition is not forbidden. (2) If one of the states is an S state, then (a) if the parities of the configurations are the same, then the other state must be an S, D, G, . state; (b) if the parities of the configurations are opposite ...
... (1) If neither of the states is an S state, then the autoionizing transition is not forbidden. (2) If one of the states is an S state, then (a) if the parities of the configurations are the same, then the other state must be an S, D, G, . state; (b) if the parities of the configurations are opposite ...
File
... • Identify the total number of Electrons -> E = _____ • Draw the Bohr Diagram • You do not need to draw the protons or neutrons inside the nucleus • You may simply leave the inside of the nucleus blank ...
... • Identify the total number of Electrons -> E = _____ • Draw the Bohr Diagram • You do not need to draw the protons or neutrons inside the nucleus • You may simply leave the inside of the nucleus blank ...
Chapter 3. The Structure of the Atom
... the atom’s mass to that of hydrogen). Since the electron was also known and measured to be much less massive than the atom, it was expected that mass of the positively charged component of the atom would be significant (relatively speaking; the atoms were known to be electrically neutral). Understan ...
... the atom’s mass to that of hydrogen). Since the electron was also known and measured to be much less massive than the atom, it was expected that mass of the positively charged component of the atom would be significant (relatively speaking; the atoms were known to be electrically neutral). Understan ...
Document
... Click any of the background top tabs to display the respective folder. Within the Chapter Outline, clicking a section tab on the right side of the screen will bring you to the first slide in each respective section. ...
... Click any of the background top tabs to display the respective folder. Within the Chapter Outline, clicking a section tab on the right side of the screen will bring you to the first slide in each respective section. ...
C. - Taylor County Schools
... Menu or any Chapter Outline slide. From within any feature, click the Resources tab to return to this slide. The “Return” button will allow you to return to the slide that you were viewing when you clicked either the Resources or Help tab. To exit the presentation, click the Exit button on the Chapt ...
... Menu or any Chapter Outline slide. From within any feature, click the Resources tab to return to this slide. The “Return” button will allow you to return to the slide that you were viewing when you clicked either the Resources or Help tab. To exit the presentation, click the Exit button on the Chapt ...
Atomic and Nuclear Physics
... state level to a lower one. The emitted energy is equal to the difference in energy between the initial and final states. Similarly, an electron can jump from one energy state level to a higher one only if it receives an amount of energy equal to the difference in energy between the final and initia ...
... state level to a lower one. The emitted energy is equal to the difference in energy between the initial and final states. Similarly, an electron can jump from one energy state level to a higher one only if it receives an amount of energy equal to the difference in energy between the final and initia ...
View - Rutgers Physics
... picture. One and one-half wavelengths are fit into the box, so the wavelength is λ = 2L/3. Since each half-wavelength corresponds to one energy level, this means the quantum energy number is 3. ...
... picture. One and one-half wavelengths are fit into the box, so the wavelength is λ = 2L/3. Since each half-wavelength corresponds to one energy level, this means the quantum energy number is 3. ...
Getting to Know Y . T ROBERT L
... early in the 1950s made it clear that these “elementary” constituents were not so elementary after all. The same signatures of compositeness have repeated every time one set of constituents is replaced by another: Excitations. If a particle is composite, when it is hit hard enough its component part ...
... early in the 1950s made it clear that these “elementary” constituents were not so elementary after all. The same signatures of compositeness have repeated every time one set of constituents is replaced by another: Excitations. If a particle is composite, when it is hit hard enough its component part ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.