Physics 513 Topic List/Study Checksheet This should function more
... calculation that could be asked from this unit?” Note that this will be similar to the middling level long answer questions from the unit exams. In general, for a final, the physics teachers believe it would not be appropriate to ask the most difficult thing from a given unit. We are focusing on cor ...
... calculation that could be asked from this unit?” Note that this will be similar to the middling level long answer questions from the unit exams. In general, for a final, the physics teachers believe it would not be appropriate to ask the most difficult thing from a given unit. We are focusing on cor ...
Answers Yr12 Physics
... The current is induced in such a direction as to oppose the field that created it. This means that the current that is induced creates its own magnetic field that repels (opposes) the increasing magnetic field strength. When the magnet is withdrawn a current is induced in the opposite direction. Thi ...
... The current is induced in such a direction as to oppose the field that created it. This means that the current that is induced creates its own magnetic field that repels (opposes) the increasing magnetic field strength. When the magnet is withdrawn a current is induced in the opposite direction. Thi ...
A = 27
... atom. If three e- were lost 10, are remaining. ANS-4 #33 The excited state must have the same # of electrons as the neutral atom, however one or more must be at a higher energy level (outermost shell) that the ground state of the periodic table ( for Al it is 2-8-3), 13 electrons.The ans is 1) 2-7-4 ...
... atom. If three e- were lost 10, are remaining. ANS-4 #33 The excited state must have the same # of electrons as the neutral atom, however one or more must be at a higher energy level (outermost shell) that the ground state of the periodic table ( for Al it is 2-8-3), 13 electrons.The ans is 1) 2-7-4 ...
Slide 1
... Jupiter rules the sky in this labeled view of a starry September night from the Alborz mountains in Iran, complete with the trail of a red flashlight illuminating the mountain ...
... Jupiter rules the sky in this labeled view of a starry September night from the Alborz mountains in Iran, complete with the trail of a red flashlight illuminating the mountain ...
Study clarifies how gamma rays generated in thunderclouds
... through. If these particles strike air molecules within the cloud, they can become 'seed particles,' sending electrons spinning off at near light speeds. Winter thunderclouds near Niigata contain powerful electric fields, with the top and bottom being positively charged and the center negatively cha ...
... through. If these particles strike air molecules within the cloud, they can become 'seed particles,' sending electrons spinning off at near light speeds. Winter thunderclouds near Niigata contain powerful electric fields, with the top and bottom being positively charged and the center negatively cha ...
Aulenbacher_EUCARD_coordination_meeting3_talk
... 14 Particpants (China, Germany, Japan, Switzerland, Russia, US,) Small, but extremely intense meeting with vivid Discussions… Highlights: Vertical polarization In fcc-ee energy calibration (Ginafelice, Hillert, Koop, Fcc-hh: polarization not hopeless (Ptytsin) internal Target also (Lenisa) ...
... 14 Particpants (China, Germany, Japan, Switzerland, Russia, US,) Small, but extremely intense meeting with vivid Discussions… Highlights: Vertical polarization In fcc-ee energy calibration (Ginafelice, Hillert, Koop, Fcc-hh: polarization not hopeless (Ptytsin) internal Target also (Lenisa) ...
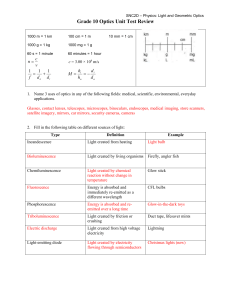
What is Refraction
... The bending of light when it travels from one material (medium) to another. What causes refraction? Refraction is caused by the speed of light changing (either slowing down or speeding up) when it enters a material that is more optically dense or less optically dense. Questions: 1. How does the spee ...
... The bending of light when it travels from one material (medium) to another. What causes refraction? Refraction is caused by the speed of light changing (either slowing down or speeding up) when it enters a material that is more optically dense or less optically dense. Questions: 1. How does the spee ...
Session Objectives
... Final kinetic energy = Initial kinetic energy + Loss in potential energy Initial KE in all the cases are equal. Loss in PE = mgh is also equal in all the cases. ...
... Final kinetic energy = Initial kinetic energy + Loss in potential energy Initial KE in all the cases are equal. Loss in PE = mgh is also equal in all the cases. ...
light1
... atom through the bulk of the material; rather the electrons vibrate for short periods of time and then reemit the energy as a reflected light wave Metals are opaque- free electrons will vibrate easily and reflects light…. This is why metals are shiny ...
... atom through the bulk of the material; rather the electrons vibrate for short periods of time and then reemit the energy as a reflected light wave Metals are opaque- free electrons will vibrate easily and reflects light…. This is why metals are shiny ...