* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Name
Photoelectric effect wikipedia , lookup
Nuclear structure wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Density of states wikipedia , lookup
Internal energy wikipedia , lookup
Gibbs free energy wikipedia , lookup
Eigenstate thermalization hypothesis wikipedia , lookup
Centripetal force wikipedia , lookup
Newton's laws of motion wikipedia , lookup
Classical central-force problem wikipedia , lookup
Work (thermodynamics) wikipedia , lookup
Kinetic energy wikipedia , lookup
Relativistic mechanics wikipedia , lookup
Hunting oscillation wikipedia , lookup
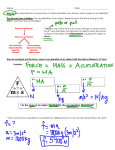
Name: ______________________________ Date: ____________ Period: ____ Physics Test Study Guide Objective Ⓡ 8.6(A) Unbalanced Forces: Students will be able to demonstrate and calculate how unbalanced forces change the speed or direction of an object's motion. Ⓡ 8.6(B) Speed, Velocity, Acceleration: Students will be able to differentiate between speed, velocity, and acceleration. Ⓡ 8.6(C) Newton’s Laws: Students will be able to investigate and describe applications of Newton's law of inertia, law of force and acceleration, and law of action-reaction. Ⓢ 6.8(A) Potential & Kinetic: Students will be able to compare and contrast potential and kinetic energy. Ⓢ 6.8(C) Speed: Students will be able to calculate average speed using distance and time measurements. Ⓢ 6.8(D) Motion Graphs: Students will be able to measure and graph changes in motion. Ⓢ 6.9(C) Energy Transformations: Students will be able to demonstrate energy transformations such as energy in a flashlight battery changes from chemical energy to electrical energy to light energy. Ⓢ 7.7(A) Work: Contrast situations where work is done with different amounts of force to situations where no work is done such as moving a box with a ramp and without a ramp, or standing still. Due: ___________ Test on: ______________ # of Questions 4 5 7 Ch. 5.2 and notes Ch. 4.1 and 4.2, notes, labs Ch. 5.2, 5.2, 5.3 and notes Notes 3 3 3 Ch. 4.1 and notes Ch. 4.1, 4.2, and notes notes 6 notes 3 In addition to completing the study guide, you need to go back through your notes and review all of the information and make sure you understand it. 1. What is speed? _______________________________________________________________________ 2. What is the difference between speed and velocity? _________________________________________ 3. What is acceleration and what causes it? a. Definition – ______________________________________________________________________ b. What causes acceleration? _________________________________________________________ 4. How do you calculate the average speed of an object? (Hint: it is a formula) _______________________ 5. What is a train’s average speed if it travels 100 kilometers in half of an hour? Make sure to include units and show your work. Use the graph on the right to answer questions 6 - 8. 6. What object would most likely be represented by the graph? 7. Label what is happening at each position on the graph. Constant velocity (+ / - CV), rest (R), or acceleration (A). A. Calculate the speed between 0s and 4s? _____________ B. Calculate the speed between 4s and 6s? _____________ C. Calculate the speed between 10s and 12s? ____________ 8. What is the average speed of the object? __________________________________ Use the graph to the left to answer questions 9 - 11. 9. Label what the object is doing at each point on the graph? Constant velocity (CV), stopped (R), positive acceleration(+A), or negative acceleration(-A). 10. What is the acceleration of the object represented by graph from 2 s – 4s? Make sure to include units and show your work. 11. What is the acceleration of the object represented by graph from 4 s – 7s? Make sure to include units and show your work. 12. A passenger riding in a train without a seat belt will continue moving forward if the train suddenly slams on the breaks. a. Explain why this will happen: ________________________________________________________ b. The scenario above is an example of which of Newton’s Laws? ___________ For questions 13 - 16, classify the following examples as having potential energy, kinetic energy, both potential energy and kinetic energy, or neither potential energy nor kinetic energy. 13. A soccer ball rolling half-way toward the goal. ____________________________________________ 14. Electricity flowing through a circuit to turn a light bulb on. ___________________________________ 15. A person pouring a glass of water from a pitcher right before it hits the glass. ____________________ 16. A person about to shoot an arrow from a bow. _____________________________________________ 17. Give an example of electrical energy being converted into thermal (heat) energy. ______________________________________________________________________________________ 18. Jose is pushing a box up a 5 meter hill with a force of 20 Newtons. How much work is Jose doing? Draw a picture, write the equation, fill in with units, and answer with units. 19. At what point does the roller coaster have a. the most potential energy? ________ b. the most kinetic energy? ________ 20. Jack is pulling on a rope with a force of 20 Newtons. Jill is pulling on the same rope with a force of 15 Newtons. a. Draw a diagram of the situation. Label the forces and the direction of force. b. What is the net force acting on the rope? Make sure to include units and show your work. c. Will the rope move at a constant speed, increasing speed, or decreasing speed toward Jack? 21. What is mechanical energy? Give an example. _______________________________________________ ________________________________________________________________________________________ 22. Maria is pushing a chair with a force of 10 Newtons. Bianca is pulling on the same chair with a force of 8 Newtons. a. Draw a diagram of the situation. Label the forces and the direction of force. b. What is the net force acting on the chair? Make sure to include units and show your work. 23. Mike kicks a soccer ball with a force of 68 Newtons and does 100 Joules of work. How far did Mike kick the soccer ball? Make sure to include units and show your work. 24. Label the following as Work or No Work: ______ a. You sit in a chair and hold a ball. ______ b. You hold a ball 2m above the floor. ______c. You lift the ball 1 m form the ground to a table. 25. A skate boarder pushes off of a wall. Draw a picture and label the forces. a. What is the action force? __________________________ b. What is the reaction force? ________________________ c. Which of Newton’s Laws does this situation represent? ______ 26. List the energy conversions that occur when you heat up a bbq pit with a propane gas tank. _________________________________ → ____________________________________ Use Newton’s 2nd law of motion to solve the following problems. Draw and label a picture. Show your work. 2 27. A 10 kg bowling ball would require what force to accelerate down an alleyway at a rate of 3 m/s ? 2 28. What is the mass of a truck if it produces a force of 14,000 N while accelerating at a rate of 5.2 m/s ? 29. What is the acceleration of softball if it has a mass of 0.75 kg and hits the catcher's glove with a force of 35 N? 30. What is radiant energy? _______________________________________________________________ 31. Using the diagram above, list between which two steps the following energy conversions are occurring: A. Radiant energy is being converted to chemical energy __________________________ B. Chemical energy is being converted to thermal energy__________________________ C. Mechanical energy is being converted into kinetic energy ________________________ 32. Where do monkeys get most of their chemical potential energy from? __________________________ 33. What two types of energy do monkeys turn chemical potential energy into? a. Type 1 – ________________________________________________________ b. Type 2 – ________________________________________________________ Chemistry Review Questions Objective Ⓡ 8.5 (A) Atoms: Students will be able to describe the structure of atoms, including the masses, electrical charges, and locations of protons, neutrons, and electrons. Ⓡ 8.5 (B) Element Identity: Students will be able to identify that protons determine an element’s identity and valence electrons determine its chemical properties. Ⓡ 8.5 (C) Periodic Table: Students will be able to interpret the arrangement of the Periodic Table, including groups and periods. Ⓡ 8.5 (D) Chemical Formulas: Students will be able to recognize that chemical formulas are used to identify substances and determine the number of atoms of each element. Ⓡ 8.5 (E) Chemical Reactions: Students will be able to investigate how evidences of chemical reactions indicate that new substances are formed. Ⓡ 8.5 (F) Balancing Equations: Students will be able to recognize whether or not a chemical equation is balanced and how that applies to the Law of Conservation of Mass. Ⓢ 6.5(C) Elements & Compounds: Differentiate between elements and compounds on the most basic level. Ⓢ 6.6 (B) Density: Students will be able to calculate density and use density to determine if an object will sink or float. Ⓢ 6.6 (A) Physical Properties: Compare metals, nonmetals, and metalloids using physical properties such as luster, conductivity or malleability. Ⓢ 7.6B Physical & Chemical Changes: Distinguish between physical and chemical changes in matter in the digestive system. 1. What are the elements in the chemical formula 3Ca(OH)2?______________________________________ 2. Determine the number of atoms of each element in the chemical formula Al2(SO4)3 Al - ______ S - _______ O - _______ Classify each of the following as a physical (P) or chemical (C) change. 3. 4. 5. 6. Aluminum bubbles and turns brown after drops of copper chloride are places on it. Mixing blue and yellow water to make green water. A plant turns sunlight, CO2, and H2O into glucose and O2 through photosynthesis. The shape of paper changes after you crumble it into a ball. _____ _____ _____ _____ 7. How many protons, neutrons, and electrons are in an atom of Aluminum? What is the atomic number and atomic mass of Aluminum? P= __________ N=___________ E=__________ Atomic #=__________ Atomic Mass=___________ 8. Circle the equation that is balanced? NH3 + H2SO4 (NH4)2SO4 2K + Cl2 2KCl N2 + F2 NF3 9. How many neutrons are in a neutral atom of an element with 22 protons, 22 electrons and an atomic mass of 48? __________________ 10. Elements in the same family (group) have ________________ properties, which are determined by their same number of ______________________ ________________________. 11. Elements can always be identified by their number of _______________. 12. Circle all the letters that apply. a. Which element has the greatest atomic mass? b. Which elements do not conduct heat and electricity? c. Which elements have properties of both metal and non-metal? d. Which elements are highly reactive metals? e. Which elements are the least reactive? f. Which elements are malleable? 13. A A A A A A B B B B B B C C C C C C D D D D D D E E E E E E F F F F F F G G G G G G Draw the Bohr model for Sodium (Na) and Fluorine (F). Put numbers and symbols for protons and neutrons and label all the electrons. Sodium # protons _____ # neutrons _____ # electrons _____ # valence electrons_____ Fluorine # protons_____ # neutrons _____ # electrons _____ # valence electrons _____ 14. A cup of gold colored metal beads was measured to have a mass of 425 grams. By water displacement, the volume of the beads was calculated to be 48.0 ml. Given the following densities, identify the metal. _________________ 15. Complete the following chart for the 3 particles that make up an atom: Sub-atomic particle Proton Neutron Electron Charge Mass 16. Where is most of an atom’s mass located? ________________________ Location in the atom