Ka and Kb from pH and Conductivity Measurements
... problems in which the acid ionization constant of a weak acid, Ka, is calculated from a given formal concentration and the pH of the solution. This approach is rarely used in the associated laboratory course. Instead, Ka is typically determined from an acid-base titration curve.1-3 If an acid or bas ...
... problems in which the acid ionization constant of a weak acid, Ka, is calculated from a given formal concentration and the pH of the solution. This approach is rarely used in the associated laboratory course. Instead, Ka is typically determined from an acid-base titration curve.1-3 If an acid or bas ...
Citric Acid Cycle
... The Citric Acid Cycle Problems: 2. What statement is NOT correct about the citrate synthase reaction in the citric acid cycle? A) its products include coenzyme A and citrate B) it forms a tricarboxylic acid C) its substrates include acetyl-CoA and oxaloacetate D) it is coupled to the hydrolysis of ...
... The Citric Acid Cycle Problems: 2. What statement is NOT correct about the citrate synthase reaction in the citric acid cycle? A) its products include coenzyme A and citrate B) it forms a tricarboxylic acid C) its substrates include acetyl-CoA and oxaloacetate D) it is coupled to the hydrolysis of ...
Structural transformation in formic acid on ultra cold ice surfaces
... light of these observations we can conclude that at 18 K the acid molecules exist in the form of dimer along with smaller fraction of the crystalline phase. As the temperature increases, the fraction of the crystalline phase increases. At 98 K a large percentage of molecules have come to the crystal ...
... light of these observations we can conclude that at 18 K the acid molecules exist in the form of dimer along with smaller fraction of the crystalline phase. As the temperature increases, the fraction of the crystalline phase increases. At 98 K a large percentage of molecules have come to the crystal ...
Chapter 26 Nutrition and Metabolism *Lecture PowerPoint
... and occupy more space in the tissues, and fat is hydrophobic, contains almost no water, and is a more compact energy storage substance • Fat is less oxidized than carbohydrates and contains over twice as much energy: 9 kcal/g for fat; 4 kcal/g for carbohydrates ...
... and occupy more space in the tissues, and fat is hydrophobic, contains almost no water, and is a more compact energy storage substance • Fat is less oxidized than carbohydrates and contains over twice as much energy: 9 kcal/g for fat; 4 kcal/g for carbohydrates ...
CHEM1611 2014-J-9 June 2014 • Alanine ( ala) and lysine (lys) are
... Consequence for structure: This rigidity and the charge on the oxygen are ideal for the formation of α-helices and β -pleated sheets via H-bonding. Consequence for chemistry: The involvement of the N lone pair in resonance, means that the N is unavailable for protonation and is non-basic. The peptid ...
... Consequence for structure: This rigidity and the charge on the oxygen are ideal for the formation of α-helices and β -pleated sheets via H-bonding. Consequence for chemistry: The involvement of the N lone pair in resonance, means that the N is unavailable for protonation and is non-basic. The peptid ...
Biochemistry Objectives 43
... a. Ebb phase (acute response): think of this phase as an urgent call for glucose and fuel use; glucagon, cortisol, epinephrine, and thyroid hormone (increased metabolism) increase while insulin decreases. Also, ADH and aldosterone increase to control fluids and sodium, respectively, while sex hormon ...
... a. Ebb phase (acute response): think of this phase as an urgent call for glucose and fuel use; glucagon, cortisol, epinephrine, and thyroid hormone (increased metabolism) increase while insulin decreases. Also, ADH and aldosterone increase to control fluids and sodium, respectively, while sex hormon ...
$doc.title
... turns yellow. What conclusion is consistent with these observations? b. The bacteria can’t ferment sucrose because they lack an enzyme to digest it. ...
... turns yellow. What conclusion is consistent with these observations? b. The bacteria can’t ferment sucrose because they lack an enzyme to digest it. ...
Plasma free amino acid profiles of canine mammary gland tumors
... TAAs were significantly decreased in the NM and M groups compared to those of the control dogs. TAA levels of the M group were lower than those of the NM group. In both the NM and M animals, plasma EAA concentrations were lower than those of the control group, and the EAA levels of the M group were ...
... TAAs were significantly decreased in the NM and M groups compared to those of the control dogs. TAA levels of the M group were lower than those of the NM group. In both the NM and M animals, plasma EAA concentrations were lower than those of the control group, and the EAA levels of the M group were ...
Polyunsaturated Fatty Acids and Cardiovascular
... netic components, including gender, age, hypertension, hyperlipidemia, obesity, and diabetes. Collectively, these have come to be known as ‘conventional’ risk factors. The importance of genetics in CVD has been examined in many family and twin studies where heritability estimates for atherosclerosis ...
... netic components, including gender, age, hypertension, hyperlipidemia, obesity, and diabetes. Collectively, these have come to be known as ‘conventional’ risk factors. The importance of genetics in CVD has been examined in many family and twin studies where heritability estimates for atherosclerosis ...
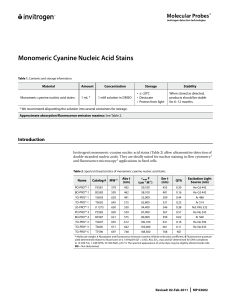
Monomeric Cyanine Nucleic Acid Stains
... optical filter sets and laser line sources suitable for excitation and detection of these dyes. These dyes may also be used with ultraviolet trans- or epi-illuminator excitation sources. As shown in Figure 2, the fluorescence excitation spectrum of DNA-bound TO-PRO®-1 dye has a short-wavelength pea ...
... optical filter sets and laser line sources suitable for excitation and detection of these dyes. These dyes may also be used with ultraviolet trans- or epi-illuminator excitation sources. As shown in Figure 2, the fluorescence excitation spectrum of DNA-bound TO-PRO®-1 dye has a short-wavelength pea ...
The Importance of Fiber in Deer Diets
... in the rumen long enough to be fermented, which is why supplemental hay and grass (or natural browse) can be beneficial. Particle size also impacts rumen muscle stimulation. The rumen tissue is partially comprised of muscle that is constantly contracting and relaxing, allowing for proper mixing of f ...
... in the rumen long enough to be fermented, which is why supplemental hay and grass (or natural browse) can be beneficial. Particle size also impacts rumen muscle stimulation. The rumen tissue is partially comprised of muscle that is constantly contracting and relaxing, allowing for proper mixing of f ...
Chapter 26
... The effect of the 5-fluoro substitution on the mechanism of action of thymidylate synthase. An enzyme thiol group (from a Cys side chain) ordinarily attacks the 6position of dUMP so that C-5 can react as a carbanion with N5,N10-methylene-THF. Normally, free enzyme is regenerated following release of ...
... The effect of the 5-fluoro substitution on the mechanism of action of thymidylate synthase. An enzyme thiol group (from a Cys side chain) ordinarily attacks the 6position of dUMP so that C-5 can react as a carbanion with N5,N10-methylene-THF. Normally, free enzyme is regenerated following release of ...
Translation
... Warm Up- You and your partner will discuss DNA replication vs transcription after watching the 2 videos DNA Replication Transcription/Translation Video ...
... Warm Up- You and your partner will discuss DNA replication vs transcription after watching the 2 videos DNA Replication Transcription/Translation Video ...
Biochemical Thermodynamics
... unavailable except on the ends Side-chain reactivity available but with slightly modified pKas. Terminal main-chain pKavalues modified too Environment of protein side chain is often hydrophobic, unlike free amino acid side chain ...
... unavailable except on the ends Side-chain reactivity available but with slightly modified pKas. Terminal main-chain pKavalues modified too Environment of protein side chain is often hydrophobic, unlike free amino acid side chain ...
Structural Studies on Sulfated Glycopeptides from the Carbohydrate
... 20 and 30 h. Following incubation the sample was lyophilized, mixed with 1 ml of 5% trichloroacetic acid, and centrifuged in a Beckman microcentrifuge for 10 min. The precipitate was washed with 0.3 ml of 5% trichloroacetic acid three times and thecombined supernatant fluid was chromatographed on Se ...
... 20 and 30 h. Following incubation the sample was lyophilized, mixed with 1 ml of 5% trichloroacetic acid, and centrifuged in a Beckman microcentrifuge for 10 min. The precipitate was washed with 0.3 ml of 5% trichloroacetic acid three times and thecombined supernatant fluid was chromatographed on Se ...
How Cell Harvest Energy
... 23. Explain why respiration is considered exergonic. __________________________________________________________________________ __________________________________________________________________________ 24. What is the main reason energy is harvested in stages in respiration ________________________ ...
... 23. Explain why respiration is considered exergonic. __________________________________________________________________________ __________________________________________________________________________ 24. What is the main reason energy is harvested in stages in respiration ________________________ ...
Quantitative analysis of complex amino acids and RGD peptides by
... 102 eV is indicative of SiO species, such as those found in siloxanes, Si(R)O, where R is a hydrocarbon chain.[61] Siloxanes are a common trace impurity with organic compounds, usually at low enough bulk levels to be insignificant for bulk properties. However, they have a low solubility in the cry ...
... 102 eV is indicative of SiO species, such as those found in siloxanes, Si(R)O, where R is a hydrocarbon chain.[61] Siloxanes are a common trace impurity with organic compounds, usually at low enough bulk levels to be insignificant for bulk properties. However, they have a low solubility in the cry ...
Krebs Cycle - ScienceFolks
... Recall that glycolysis, stage I of cellular respiration, produces two molecules of pyruvate. These molecules enter the matrix of a mitochondrion, where they start the Krebs cycle. The reactions that occur next are shown in Figure 1.1. You can watch an animated version at this link: http://www.youtub ...
... Recall that glycolysis, stage I of cellular respiration, produces two molecules of pyruvate. These molecules enter the matrix of a mitochondrion, where they start the Krebs cycle. The reactions that occur next are shown in Figure 1.1. You can watch an animated version at this link: http://www.youtub ...
vitamins ( PPT )
... Sources in diet - Many plants (photoreceptors), also meat, especially liver. Fat soluble, so you can get too much, or too little if absorption is a problem. Some uses: Vision (11-cis-retinol bound to rhodopsin detects light in our eyes). Regulating gene transcription (retinoic acid receptors on cell ...
... Sources in diet - Many plants (photoreceptors), also meat, especially liver. Fat soluble, so you can get too much, or too little if absorption is a problem. Some uses: Vision (11-cis-retinol bound to rhodopsin detects light in our eyes). Regulating gene transcription (retinoic acid receptors on cell ...
chapt08
... b. In the preparatory (prep) reaction, pyruvate enters a mitochondrion and is oxidized to a twocarbon acetyl group and CO2 is removed; this reaction occurs twice per glucose molecule. c. The citric acid cycle: 1) occurs in the matrix of the mitochondrion and produces NADH and FADH 2; 2) is a series ...
... b. In the preparatory (prep) reaction, pyruvate enters a mitochondrion and is oxidized to a twocarbon acetyl group and CO2 is removed; this reaction occurs twice per glucose molecule. c. The citric acid cycle: 1) occurs in the matrix of the mitochondrion and produces NADH and FADH 2; 2) is a series ...
pH Homeostasis in Lactic Acid Bacteria
... the medium decreases because of the accumulation of organic acids, primarily lactic acid. However, the pH within the cytoplasm of fermenting lactic acid bacteria remains more alkaline than the medium surrounding the cells (41), largely because the cells rapidly excrete protonated lactic acid, via a ...
... the medium decreases because of the accumulation of organic acids, primarily lactic acid. However, the pH within the cytoplasm of fermenting lactic acid bacteria remains more alkaline than the medium surrounding the cells (41), largely because the cells rapidly excrete protonated lactic acid, via a ...
Fermentation of sugars and fermentative enzymes
... Poisons have also been used in other ways in work on enzymes. As I stated, fermentation from sugar to alcohol and carbon dioxide passes through a considerable number of intermediate stages, amongst which each has its own enzyme. These partial reactions are so interwoven that it is difficult to separ ...
... Poisons have also been used in other ways in work on enzymes. As I stated, fermentation from sugar to alcohol and carbon dioxide passes through a considerable number of intermediate stages, amongst which each has its own enzyme. These partial reactions are so interwoven that it is difficult to separ ...
Jeopardy 2
... during fermentation that allows cells to continue to make ATP using glycolysis when oxygen is low? A: What is NAD+? S2C06 Jeopardy Review Image modified from: Pearson Education Inc; publishing as Pearson Prenctice Hall © 2006 ...
... during fermentation that allows cells to continue to make ATP using glycolysis when oxygen is low? A: What is NAD+? S2C06 Jeopardy Review Image modified from: Pearson Education Inc; publishing as Pearson Prenctice Hall © 2006 ...
Lecture Slides
... • Autotrophs are producers because ecosystems depend upon them for food. • Heterotrophs are consumers because they eat plants or other animals. ...
... • Autotrophs are producers because ecosystems depend upon them for food. • Heterotrophs are consumers because they eat plants or other animals. ...
Slide 1
... in the mitochondrion is organized into two independent linear branches Branch 1 begins with the reductive carboxylation of 2-oxoglutarate to isocitrate, which is then isomerized to citrate. This citrate is cleaved into a C2 compound and oxaloacetate, which is reduced to malate (red in figure) Br ...
... in the mitochondrion is organized into two independent linear branches Branch 1 begins with the reductive carboxylation of 2-oxoglutarate to isocitrate, which is then isomerized to citrate. This citrate is cleaved into a C2 compound and oxaloacetate, which is reduced to malate (red in figure) Br ...
Butyric acid
Butyric acid (from Greek βούτῡρον, meaning ""butter""), also known under the systematic name butanoic acid, abbreviated BTA, is a carboxylic acid with the structural formula CH3CH2CH2-COOH. Salts and esters of butyric acid are known as butyrates or butanoates. Butyric acid is found in milk, especially goat, sheep and buffalo milk, butter, parmesan cheese, and as a product of anaerobic fermentation (including in the colon and as body odor). It has an unpleasant smell and acrid taste, with a sweetish aftertaste (similar to ether). It can be detected by mammals with good scent detection abilities (such as dogs) at 10 parts per billion, whereas humans can detect it in concentrations above 10 parts per million.Butyric acid is present in, and is the main distinctive smell of, human vomit.Butyric acid was first observed (in impure form) in 1814 by the French chemist Michel Eugène Chevreul. By 1818, he had purified it sufficiently to characterize it. The name of butyric acid comes from the Latin word for butter, butyrum (or buturum), the substance in which butyric acid was first found.