Study Guide 1st Semester
... 32. Where are the alkali metal elements found? How do their electron configurations end? What are some typical behaviors of alkali metals? 33. Where are the alkaline earth metals found? How do their electron configurations end? What are some typical behaviors of alkaline earth metals? 34. What is a ...
... 32. Where are the alkali metal elements found? How do their electron configurations end? What are some typical behaviors of alkali metals? 33. Where are the alkaline earth metals found? How do their electron configurations end? What are some typical behaviors of alkaline earth metals? 34. What is a ...
Lecture 12
... moments with the electromagnetic fields of the electrons. The level splitting caused by this interaction is even smaller than the fine structure, so it is called hyperfine structure. Hyperfine states that are split from the ground state make particularly good qubits for quantum information due to th ...
... moments with the electromagnetic fields of the electrons. The level splitting caused by this interaction is even smaller than the fine structure, so it is called hyperfine structure. Hyperfine states that are split from the ground state make particularly good qubits for quantum information due to th ...
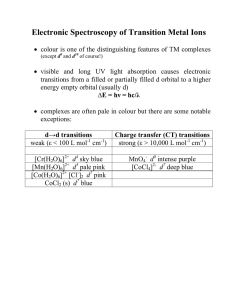
Electronic Spectroscopy of Transition Metal Ions
... in order to understand the spectroscopy of d ions with more than one d electron we must take the effect of e- - e- repulsion into account (we have ignored this so far) ...
... in order to understand the spectroscopy of d ions with more than one d electron we must take the effect of e- - e- repulsion into account (we have ignored this so far) ...
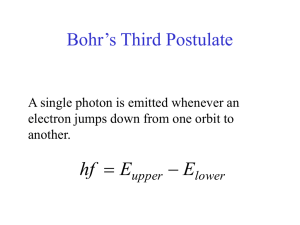
Bohr´s Third Postulate
... don’t all the photoelectrons have the same kinetic energy when they leave the metal’s surface? 4. What property of the emitted electrons depends on the intensity of incident light?What property of the emitted photoelectrons depends on the frequency of incident light? ...
... don’t all the photoelectrons have the same kinetic energy when they leave the metal’s surface? 4. What property of the emitted electrons depends on the intensity of incident light?What property of the emitted photoelectrons depends on the frequency of incident light? ...
Elements PPT
... the second can hold eight so it needs two more to be stable, that means that oxygen wants to combine with other elements or itself. ...
... the second can hold eight so it needs two more to be stable, that means that oxygen wants to combine with other elements or itself. ...
Atomic structure review
... Atomic structure review - H People Thompson – discovered electrons Rutherford – discovered the nucleus – small dense positive nucleus, volume empty space Bohr – electrons have quantized (specific) energy, shell model Heisenberg – due to wave nature of electrons you can’t know the position and moment ...
... Atomic structure review - H People Thompson – discovered electrons Rutherford – discovered the nucleus – small dense positive nucleus, volume empty space Bohr – electrons have quantized (specific) energy, shell model Heisenberg – due to wave nature of electrons you can’t know the position and moment ...
Table showing examples of Complex ions with their bond
... This is because each ion absorbs light of certain wavelengths only in the visible past of the spectrum, thus changing incident white light into light whose hue () is composed of the colours complementary those which have absorbed. The d-level is split into two when a complex ion is formed and diffe ...
... This is because each ion absorbs light of certain wavelengths only in the visible past of the spectrum, thus changing incident white light into light whose hue () is composed of the colours complementary those which have absorbed. The d-level is split into two when a complex ion is formed and diffe ...
Part 1 Electron Arrangement
... electrons could be particles yet they gave off waves of light. • De Broglie suggested that electrons could be considered waves confined to space around a nucleus only at specific frequencies. • Diffraction experiments proved that electron beams can interfere with each other and produce areas of low ...
... electrons could be particles yet they gave off waves of light. • De Broglie suggested that electrons could be considered waves confined to space around a nucleus only at specific frequencies. • Diffraction experiments proved that electron beams can interfere with each other and produce areas of low ...
Chapter 8: Periodic Properties of the Elements
... a. Ei of Be is larger than B and that of Mg is larger than Al. An explanation is that Be and Mg lose an s2 electron while B and Al are losing the p1 electron. An s electron spends more time closer to the nucleus and is therefore harder to remove. Additionally, the p electrons are shielded somewhat b ...
... a. Ei of Be is larger than B and that of Mg is larger than Al. An explanation is that Be and Mg lose an s2 electron while B and Al are losing the p1 electron. An s electron spends more time closer to the nucleus and is therefore harder to remove. Additionally, the p electrons are shielded somewhat b ...
Lecture 1 Atomic Structure
... Q: We previously mentioned that Zeff increases across the period. However, going from C to N, the increase in Zeff is 0.69, while going from N to O, the increase in Zeff is only 0.62. Why? A: From C to N, electrons add into an empty p orbital. From N to O, the eadd into occupied p orbital. There is ...
... Q: We previously mentioned that Zeff increases across the period. However, going from C to N, the increase in Zeff is 0.69, while going from N to O, the increase in Zeff is only 0.62. Why? A: From C to N, electrons add into an empty p orbital. From N to O, the eadd into occupied p orbital. There is ...
5 Electrons in Atoms
... Electron Configuration (continued) exclusion principle, the aufbau principle, and Hund's rule to write out the electron configuration and draw the orbital diagram for each of the following elements. ...
... Electron Configuration (continued) exclusion principle, the aufbau principle, and Hund's rule to write out the electron configuration and draw the orbital diagram for each of the following elements. ...
Chapter Summary
... an atom can have the same set of four quantum numbers. The layout of the periodic table of the elements has to do with the highest-energy filled or partly filled subshell in the ground-state electron configuration of an atom. Elements that have similar ground-state configurations (such as four elect ...
... an atom can have the same set of four quantum numbers. The layout of the periodic table of the elements has to do with the highest-energy filled or partly filled subshell in the ground-state electron configuration of an atom. Elements that have similar ground-state configurations (such as four elect ...
Chemistry Science Notebook
... Compare and contrast Einstein’s equation with Planck’s equation by completing the following sentence. ...
... Compare and contrast Einstein’s equation with Planck’s equation by completing the following sentence. ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.