Name: Period:______ PHYSICAL SCIENCE 1st Semester Final
... The close match between Mendeleev’s predictions and the actual properties of new elements showed how useful his periodic table could be. In the modern periodic table, elements are arranged by increasing atomic number (number of protons). Each row on the table is a period. Each column on the ...
... The close match between Mendeleev’s predictions and the actual properties of new elements showed how useful his periodic table could be. In the modern periodic table, elements are arranged by increasing atomic number (number of protons). Each row on the table is a period. Each column on the ...
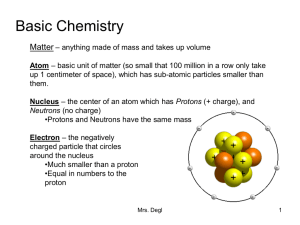
Basic Chemistry
... •The symbols have either 1 or 2 letters •The atomic number is the number of protons •They are listed on the Period Table of Elements ...
... •The symbols have either 1 or 2 letters •The atomic number is the number of protons •They are listed on the Period Table of Elements ...
Chemistry Notes with Blanks
... with the coal? The elements (carbon in coal; hydrogen and oxygen in water) only combine as sugar when _________bonds form Sugar cannot be easily separated into its components. So…Can you break gold down into a simpler substance??? NO…because it is an element Atoms are the basic building blocks of al ...
... with the coal? The elements (carbon in coal; hydrogen and oxygen in water) only combine as sugar when _________bonds form Sugar cannot be easily separated into its components. So…Can you break gold down into a simpler substance??? NO…because it is an element Atoms are the basic building blocks of al ...
Ch. 2 Chemistry
... (b) An electron can move from one level to another only if the energy it gains or loses is exactly equal to the difference in energy between the two levels. Arrows indicate some of the step-wise changes in potential energy that are possible. Copyright © 2004 Pearson Education, Inc. publishing as Ben ...
... (b) An electron can move from one level to another only if the energy it gains or loses is exactly equal to the difference in energy between the two levels. Arrows indicate some of the step-wise changes in potential energy that are possible. Copyright © 2004 Pearson Education, Inc. publishing as Ben ...
Exercises - Galena Park ISD
... 33. Explain why an electron in a low-energy orbit does not spiral into the nucleus. The possible energy levels for the electron are quantized. To spiral into the nucleus, the electron would have to pass through energy states that are less than that of its lowest energy orbit. Such orbits cannot exis ...
... 33. Explain why an electron in a low-energy orbit does not spiral into the nucleus. The possible energy levels for the electron are quantized. To spiral into the nucleus, the electron would have to pass through energy states that are less than that of its lowest energy orbit. Such orbits cannot exis ...
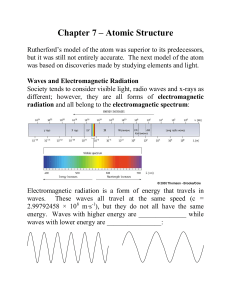
Chapter 8 - Clayton State University
... Quantum mechanics, on the other hand deals with all objects. However, for large objects the rules of quantum mechanics are so similar to Newton’s laws of motion that there is usually no need to use the more complicated concepts of quantum mechanics to describe the behavior of the objects. For object ...
... Quantum mechanics, on the other hand deals with all objects. However, for large objects the rules of quantum mechanics are so similar to Newton’s laws of motion that there is usually no need to use the more complicated concepts of quantum mechanics to describe the behavior of the objects. For object ...
Topic 3&4 Atoms and the per.table
... (c) How many (i) protons (ii) neutrons (ii) 14 (iii) electrons (iii) 13 are there in this atom? (d) Same number of protons (+ ) and (d) Explain why this atom is neutral. electrons (-) Standard Grade Chemistry ...
... (c) How many (i) protons (ii) neutrons (ii) 14 (iii) electrons (iii) 13 are there in this atom? (d) Same number of protons (+ ) and (d) Explain why this atom is neutral. electrons (-) Standard Grade Chemistry ...
Atomic Spectroscopy and the Bohr Model
... emit the energy in the form of light energy (photons). • If we slow down this light using a prism or spectrometer, we can see the constituent colors that make up the color light that we are seeing. This series of lines is called the emission spectrum. This bright line spectrum is used to identify el ...
... emit the energy in the form of light energy (photons). • If we slow down this light using a prism or spectrometer, we can see the constituent colors that make up the color light that we are seeing. This series of lines is called the emission spectrum. This bright line spectrum is used to identify el ...
Test Review: Unit 1 - Ms. Hill`s Pre
... normal chemical and physical changes (Dalton didn’t describe/clarify normal circumstances, matter can be created and destroyed in nuclear reactions) b. Law of Definite Proportions: the fact that a chemical compound contain exactly the same elements in exactly the same proportions in exactly the same ...
... normal chemical and physical changes (Dalton didn’t describe/clarify normal circumstances, matter can be created and destroyed in nuclear reactions) b. Law of Definite Proportions: the fact that a chemical compound contain exactly the same elements in exactly the same proportions in exactly the same ...
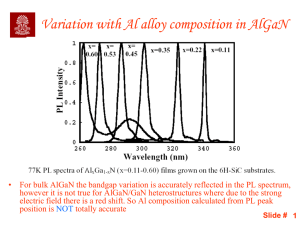
Slide 1
... • For bulk AlGaN the bandgap variation is accurately reflected in the PL spectrum, however it is not true for AlGaN/GaN heterostructures where due to the strong electric field there is a red shift. So Al composition calculated from PL peak position is NOT totally accurate ...
... • For bulk AlGaN the bandgap variation is accurately reflected in the PL spectrum, however it is not true for AlGaN/GaN heterostructures where due to the strong electric field there is a red shift. So Al composition calculated from PL peak position is NOT totally accurate ...
7Copenhagen
... electron went through with light beam (particle behaviour) •If interference pattern appears, then we have both wave and particle behaviour •Complementarity says it must be either ...
... electron went through with light beam (particle behaviour) •If interference pattern appears, then we have both wave and particle behaviour •Complementarity says it must be either ...
Column A
... d. How is the number of protons determined? From the atomic number e. How is the number of neutrons determined? Mass # - # of protons f. How is the number of electrons determined in a neutral atom? # protons = # electrons g. What subatomic particles are located in the nucleus? Protons and neutrons h ...
... d. How is the number of protons determined? From the atomic number e. How is the number of neutrons determined? Mass # - # of protons f. How is the number of electrons determined in a neutral atom? # protons = # electrons g. What subatomic particles are located in the nucleus? Protons and neutrons h ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.