
The Electronic Structures of Atoms Electromagnetic Radiation The
... In 1913 Neils Bohr incorporated Planck’s quantum theory into the hydrogen spectrum explanation. Here are the postulates of Bohr’s theory. Atom has a number of definite and discrete energy levels (orbits) in which an electron may exist without emitting or absorbing electromagnetic radiation. As the o ...
... In 1913 Neils Bohr incorporated Planck’s quantum theory into the hydrogen spectrum explanation. Here are the postulates of Bohr’s theory. Atom has a number of definite and discrete energy levels (orbits) in which an electron may exist without emitting or absorbing electromagnetic radiation. As the o ...
Pretest 4.3 2008
... How would you prepare three solutions representing the three types of electrolytes? ...
... How would you prepare three solutions representing the three types of electrolytes? ...
Photosynthesis Stores Energy in Organic Compounds
... compounds are in a low-energy state and need to be converted to a high-energy state • Compounds are activated by ATP, then reduced by ...
... compounds are in a low-energy state and need to be converted to a high-energy state • Compounds are activated by ATP, then reduced by ...
Chapter 7 Quantum Theory of the Atom
... 3s subshell with one orbital. When l = 1, ml has only three values, -1, 0, 1. So there is a 3p subshell with three orbitals. When l = 2, ml has only five values, -2, -1, 0, 1, 2. So there is a 3d subshell with five orbitals. ...
... 3s subshell with one orbital. When l = 1, ml has only three values, -1, 0, 1. So there is a 3p subshell with three orbitals. When l = 2, ml has only five values, -2, -1, 0, 1, 2. So there is a 3d subshell with five orbitals. ...
Chapter 5 Mendeleev`s Periodic Table
... The Bohr Model of the Atom • In 1913, Niels Bohr suggested a new model of the atom that explained why hydrogen had a discrete line spectrum rather than a continuous spectrum. • Bohr's basic theory: electrons in atoms can only be at certain energy levels, and they can give off or absorb radiation onl ...
... The Bohr Model of the Atom • In 1913, Niels Bohr suggested a new model of the atom that explained why hydrogen had a discrete line spectrum rather than a continuous spectrum. • Bohr's basic theory: electrons in atoms can only be at certain energy levels, and they can give off or absorb radiation onl ...
Orbitals
... spectrum of hydrogen, that calculates the energy levels an electron may have in the hydrogen atom: E=-2.178 x 10-18J(Z2/n2) Where Z = atomic number n = an integer ...
... spectrum of hydrogen, that calculates the energy levels an electron may have in the hydrogen atom: E=-2.178 x 10-18J(Z2/n2) Where Z = atomic number n = an integer ...
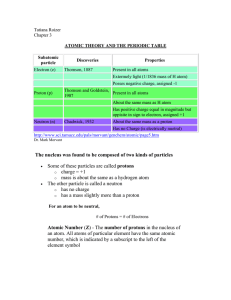
atomic theory and the periodic table
... Each orbital has a name. The orbital occupied by the hydrogen electron is called a 1s orbital. The "1" represents the fact that the orbital is in the energy level closest to the nucleus. The "s" tells you about the shape of the orbital. s orbitals are spherically symmetric around the nucleus - in e ...
... Each orbital has a name. The orbital occupied by the hydrogen electron is called a 1s orbital. The "1" represents the fact that the orbital is in the energy level closest to the nucleus. The "s" tells you about the shape of the orbital. s orbitals are spherically symmetric around the nucleus - in e ...
Energy level
... atoms. Three rules tell us how: 1) Aufbau principle - electrons enter the lowest energy first. ...
... atoms. Three rules tell us how: 1) Aufbau principle - electrons enter the lowest energy first. ...
Chapter 37 Early Quantum Theory and Models of the Atom
... Determine the wavelength of light emitted when a hydrogen atom makes a transition from the n = 6 to the n = 2 energy level according to the Bohr ...
... Determine the wavelength of light emitted when a hydrogen atom makes a transition from the n = 6 to the n = 2 energy level according to the Bohr ...
Energy
... four quantum numbers. 2) Aufbau principle - Electrons add to the lowest energy available orbital until that orbital is filled. Applies to the ground (lowest energy) state of the atom. 3) Hund’s rule - Electrons add in such a way as to make as many of the electrons as possible “spin up” (ms = 1/2). E ...
... four quantum numbers. 2) Aufbau principle - Electrons add to the lowest energy available orbital until that orbital is filled. Applies to the ground (lowest energy) state of the atom. 3) Hund’s rule - Electrons add in such a way as to make as many of the electrons as possible “spin up” (ms = 1/2). E ...
Ch 7 Lecture Notes
... Erwin Schrödinger (1887-1961), an Austrian Scientist Developed a mathematical wave function (H)(psi) to describe the ____________ for finding a given electron for the hydrogen atom in certain regions of space. The equation is long and complex, but includes _________ important variables: ...
... Erwin Schrödinger (1887-1961), an Austrian Scientist Developed a mathematical wave function (H)(psi) to describe the ____________ for finding a given electron for the hydrogen atom in certain regions of space. The equation is long and complex, but includes _________ important variables: ...
- Cronodon
... causing shifts and splitting in what appear to be single energy levels when viewed with higher resolution. The fine structure is also affected by relativistic effects – when electron speeds reach a significant fraction of the speed of light, Schrodinger’s equation becomes increasingly approximate an ...
... causing shifts and splitting in what appear to be single energy levels when viewed with higher resolution. The fine structure is also affected by relativistic effects – when electron speeds reach a significant fraction of the speed of light, Schrodinger’s equation becomes increasingly approximate an ...
L 35 Modern Physics [1]
... according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a continuous range of wavelengths (colors) NOT SO! ...
... according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a continuous range of wavelengths (colors) NOT SO! ...
Chapter 1 Atoms Properties of Matter Intensive vs. Extensive
... Atomic Number Nuclide Isotope’s definition Average atomic mass Avogadro’s Number Mole Molar Mass Chapter 4 Electromagnetic Radiation o Particles and Wave Properties of Light ...
... Atomic Number Nuclide Isotope’s definition Average atomic mass Avogadro’s Number Mole Molar Mass Chapter 4 Electromagnetic Radiation o Particles and Wave Properties of Light ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.


















![L 35 Modern Physics [1]](http://s1.studyres.com/store/data/008517000_1-9aef89c0ca089782f518550164188024-300x300.png)



