Chapter 4 Powerpoint
... 1.) The electron travels in orbits (energy levels) around the nucleus. 2.) The orbits closest to the nucleus are lowest in energy, those further out are higher in energy. 3.) When energy is absorbed by the atom, the electron moves into a higher energy orbit. This energy is released when the elec ...
... 1.) The electron travels in orbits (energy levels) around the nucleus. 2.) The orbits closest to the nucleus are lowest in energy, those further out are higher in energy. 3.) When energy is absorbed by the atom, the electron moves into a higher energy orbit. This energy is released when the elec ...
First Semester Final - Review Questions
... 37. Describe the different amounts and kinds of damage in matter produced by the different penetrations of each type of radioactive decay. 38. How does the energy release in a nuclear reaction compare to the energy release in a chemical reaction. Investigation and Experimentation 39. What is the pur ...
... 37. Describe the different amounts and kinds of damage in matter produced by the different penetrations of each type of radioactive decay. 38. How does the energy release in a nuclear reaction compare to the energy release in a chemical reaction. Investigation and Experimentation 39. What is the pur ...
pptx
... Two sets of challenges • How to best parallelize existing GW-BSE algorithms? Will rely on Charm++ to deliver high performance Coding, maintenance, migration to other computers much easier for user • Need to improve GW-BSE algorithms to use the computers more effective (theoretical physicist/chemist’ ...
... Two sets of challenges • How to best parallelize existing GW-BSE algorithms? Will rely on Charm++ to deliver high performance Coding, maintenance, migration to other computers much easier for user • Need to improve GW-BSE algorithms to use the computers more effective (theoretical physicist/chemist’ ...
Work sheet –chapter 2 CLASS - XI CHEMISTRY (Structure of Atom
... 7. Calculate energy of 2mole of photons of radiation whose frequency is 51014Hz. 8. What is emission and absorption spectra? 9. What transition in the hydrogen spectrum would have the same wavelength as the Balmer transition, n = 4 to n = 2 of He+ spectrum? 10. Spectral lines are regarded as the fi ...
... 7. Calculate energy of 2mole of photons of radiation whose frequency is 51014Hz. 8. What is emission and absorption spectra? 9. What transition in the hydrogen spectrum would have the same wavelength as the Balmer transition, n = 4 to n = 2 of He+ spectrum? 10. Spectral lines are regarded as the fi ...
Chemistry 106: General Chemistry
... I3PF5 What mass of nitrogen dioxide would be contained in a 4.32 L vessel at 48° C and 1062 torr? 5.35 x 104 g 53.5 g 10.5 g 105.0 g none of the above ...
... I3PF5 What mass of nitrogen dioxide would be contained in a 4.32 L vessel at 48° C and 1062 torr? 5.35 x 104 g 53.5 g 10.5 g 105.0 g none of the above ...
Atomic Term Symbols
... the same energy. The other members are typically linear combinations of Slater determinants. This is the first excited state of the atom. For atoms the first excited electronic state is on the order of one eV=8050 cm-1 higher in energy than the ground ...
... the same energy. The other members are typically linear combinations of Slater determinants. This is the first excited state of the atom. For atoms the first excited electronic state is on the order of one eV=8050 cm-1 higher in energy than the ground ...
Unit 4 Notes
... 1) According to the aufbau chart, copper should have this electron configuration: 2) Instead, it has this electron configuration: 3) Exceptions occur in groups 6B and 1B because: a. Sublevels are most stable when they are b. Sublevels are fairly stable when they are c. Sublevels lack stability when ...
... 1) According to the aufbau chart, copper should have this electron configuration: 2) Instead, it has this electron configuration: 3) Exceptions occur in groups 6B and 1B because: a. Sublevels are most stable when they are b. Sublevels are fairly stable when they are c. Sublevels lack stability when ...
AP Notes Chapter 7
... from each other in fundamental ways. Matter was particles. Energy could come in waves, with any frequency. Max Planck found that as the cooling of hot objects couldn’t be explained by viewing energy as a wave. ...
... from each other in fundamental ways. Matter was particles. Energy could come in waves, with any frequency. Max Planck found that as the cooling of hot objects couldn’t be explained by viewing energy as a wave. ...
Advanced Physical Chemistry
... spin-orbit coupling come about and change the spectrum . Be able to use the expression for the interaction energy, and how does it relate to the total energy. Be able to write a term symbol. What is Russel Saunders coupling, jj coupling? What is the multiplicity? Be able to write out the hamiltonian ...
... spin-orbit coupling come about and change the spectrum . Be able to use the expression for the interaction energy, and how does it relate to the total energy. Be able to write a term symbol. What is Russel Saunders coupling, jj coupling? What is the multiplicity? Be able to write out the hamiltonian ...
Exam topics-- understand and be able to apply ideas like in
... 8. Atomic spectra and connection with electron energy levels in atoms. 9. Atomic spectra as signature of atom. 10. Potential energy of electron in atom. 11. Atomic discharge lamps- how work and why so efficient at converting electrical energy to light. 12. Bohr model. What it explained and what were ...
... 8. Atomic spectra and connection with electron energy levels in atoms. 9. Atomic spectra as signature of atom. 10. Potential energy of electron in atom. 11. Atomic discharge lamps- how work and why so efficient at converting electrical energy to light. 12. Bohr model. What it explained and what were ...
Bio_130_files/Chemistry Review
... • interact with other atoms to form bonds – Electrons – have a negative charge and 1/2000 the mass of a proton (0 amu) ...
... • interact with other atoms to form bonds – Electrons – have a negative charge and 1/2000 the mass of a proton (0 amu) ...
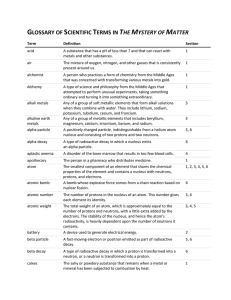
GLOSSARY OF SCIENTIFIC TERMS IN THE MYSTERY OF MATTER
... A unit that measures the effect of ionizing radiation upon a particular person. A group of two or more atoms linked together by sharing electrons in a chemical bond. A heavy, neutral particle in an atom’s nucleus that accounts for almost all of each atom’s mass, in addition to protons. Any of the si ...
... A unit that measures the effect of ionizing radiation upon a particular person. A group of two or more atoms linked together by sharing electrons in a chemical bond. A heavy, neutral particle in an atom’s nucleus that accounts for almost all of each atom’s mass, in addition to protons. Any of the si ...
Lectures 6-7 - U of L Class Index
... d orbitals (l = 2) A d orbital has ___ nodes passing through the nucleus. For four of the d orbitals, both of these nodes are planes, giving a ‘petal-shaped’ orbital. For the fifth d orbital (_____),the nodes look more like a pair of inverted cones. This gives an orbital that looks a bit like a p or ...
... d orbitals (l = 2) A d orbital has ___ nodes passing through the nucleus. For four of the d orbitals, both of these nodes are planes, giving a ‘petal-shaped’ orbital. For the fifth d orbital (_____),the nodes look more like a pair of inverted cones. This gives an orbital that looks a bit like a p or ...
CHEMICAL BONDING
... Need a new theory — now called QUANTUM or WAVE MECHANICS. e- can only exist in certain discrete orbits — called stationary states. e- is restricted to QUANTIZED energy states. ...
... Need a new theory — now called QUANTUM or WAVE MECHANICS. e- can only exist in certain discrete orbits — called stationary states. e- is restricted to QUANTIZED energy states. ...
PERIODIC TABLE OF THE ELEMENTS
... • For the first eighteen elements, there are some easy rules: – The K shell only holds two electrons. ...
... • For the first eighteen elements, there are some easy rules: – The K shell only holds two electrons. ...
Name - cloudfront.net
... Draw the molecular Lewis dot structure for the following compounds and determine the shape of the molecule. ...
... Draw the molecular Lewis dot structure for the following compounds and determine the shape of the molecule. ...
Photosynthesis Stores Energy in Organic Compounds
... Uses ATP and NADPH to reduce CO2 to make glucose, which can be converted to starch ...
... Uses ATP and NADPH to reduce CO2 to make glucose, which can be converted to starch ...
Term Symbols
... Note: Molecular orbitals have symbols corresponding to the quantum number as follows: Symbol: for 0 Symbol: for 1 Symbol: for 2 etc… Further discussions of these topics can be found in Mortimer pages 651 and 663. Term symbols can be used to determine the allowed electronic tran ...
... Note: Molecular orbitals have symbols corresponding to the quantum number as follows: Symbol: for 0 Symbol: for 1 Symbol: for 2 etc… Further discussions of these topics can be found in Mortimer pages 651 and 663. Term symbols can be used to determine the allowed electronic tran ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.