UNIT 3 VOCABULARY MATCHING and mole problems
... ____ 2.) equal to the number of protons in an atom; whole number on the Periodic Table ____ 3.) equal to the number of protons plus the number of neutrons in an atom ____ 4.) discovered the electron using a cathode ray tube ____ 5.) atoms of the same element, but have different masses ____ 6.) negat ...
... ____ 2.) equal to the number of protons in an atom; whole number on the Periodic Table ____ 3.) equal to the number of protons plus the number of neutrons in an atom ____ 4.) discovered the electron using a cathode ray tube ____ 5.) atoms of the same element, but have different masses ____ 6.) negat ...
Unit 6 Worksheet Package
... between these two types of ions forms an _____________ bond. Nearly all ionic compounds are _____________ solids at room temperature. In these solids the total _____________ charge is balanced by the total _____________ charge. Ionic compounds in general have very _____________ melting points. This ...
... between these two types of ions forms an _____________ bond. Nearly all ionic compounds are _____________ solids at room temperature. In these solids the total _____________ charge is balanced by the total _____________ charge. Ionic compounds in general have very _____________ melting points. This ...
Adding Fermi-Dirac Statistics to the Drude Model = Sommmerfield
... wrong? In particular, the electrons don’t seem to be scattered by each other. Why? Why is the actual heat capacity of metals much smaller than predicted? ...
... wrong? In particular, the electrons don’t seem to be scattered by each other. Why? Why is the actual heat capacity of metals much smaller than predicted? ...
RPA - Department of Theoretical Physics UMCS
... are just the numbers obtained from the two-dimensional oscillator with ω+ = 2 ω-. ...
... are just the numbers obtained from the two-dimensional oscillator with ω+ = 2 ω-. ...
Atomic Structure
... may have the same four quantum numbers Hund’s Rule of Maximum Multiplicity: degenerate orbitals are each singly occupied before electron-pairing can occur, and spins are “parallel” ...
... may have the same four quantum numbers Hund’s Rule of Maximum Multiplicity: degenerate orbitals are each singly occupied before electron-pairing can occur, and spins are “parallel” ...
teacher version filled in
... It is inherently impossible for us to simultaneously know both the exact momentum and exact location of an electron This is because anything we do to determine the location or momentum of the electron moves it from its original path and location; this can’t be reduced past a certain minimal level We ...
... It is inherently impossible for us to simultaneously know both the exact momentum and exact location of an electron This is because anything we do to determine the location or momentum of the electron moves it from its original path and location; this can’t be reduced past a certain minimal level We ...
Chapter 5 Electrons in Atoms
... This gives us two half filled orbitals (the others are all still full) Half full is slightly lower in energy. The same principal applies to ...
... This gives us two half filled orbitals (the others are all still full) Half full is slightly lower in energy. The same principal applies to ...
atomic number - geraldinescience
... • The number of protons in the nucleus of an atom is called the atomic number. • All atoms of any given element have the same atomic number. An element’s atomic number sets the atoms of that element apart from the atoms of all other elements. • Elements on the periodic table are ordered according to ...
... • The number of protons in the nucleus of an atom is called the atomic number. • All atoms of any given element have the same atomic number. An element’s atomic number sets the atoms of that element apart from the atoms of all other elements. • Elements on the periodic table are ordered according to ...
CHAPTER 4: Structure of the Atom
... is the number of positive charges in the nucleus Could neither account for the intensities nor the fine structure of the spectral lines (they are actually doublets) for hydrogen when atoms were put into magnetic fields (Nobel prize to Lorentz and Zeeman 1902) Could not explain the binding of atoms i ...
... is the number of positive charges in the nucleus Could neither account for the intensities nor the fine structure of the spectral lines (they are actually doublets) for hydrogen when atoms were put into magnetic fields (Nobel prize to Lorentz and Zeeman 1902) Could not explain the binding of atoms i ...
The Science and Engineering of Materials, 4th ed Donald R. Askeland
... Section 2.3 The Electronic Structure of the Atom Quantum numbers are the numbers that assign electrons in an atom to discrete energy levels. A quantum shell is a set of fixed energy levels to which electrons belong. Pauli exclusion principle specifies that no more than two electrons in a mate ...
... Section 2.3 The Electronic Structure of the Atom Quantum numbers are the numbers that assign electrons in an atom to discrete energy levels. A quantum shell is a set of fixed energy levels to which electrons belong. Pauli exclusion principle specifies that no more than two electrons in a mate ...
Chapter 28
... • There are two directions for the spin: spin up, ms = ½; spin down, ms = - ½ • There is a slight energy difference between the two spins and this accounts for the doublet in some lines • A classical description of electron spin is incorrect: the electron cannot be located precisely in space, thus i ...
... • There are two directions for the spin: spin up, ms = ½; spin down, ms = - ½ • There is a slight energy difference between the two spins and this accounts for the doublet in some lines • A classical description of electron spin is incorrect: the electron cannot be located precisely in space, thus i ...
Atomic Structure (history of atom)
... John Dalton performed experiments to study the ratio in which elements combine in chemical reactions He then formulated a hypotheses and theories to explain his ...
... John Dalton performed experiments to study the ratio in which elements combine in chemical reactions He then formulated a hypotheses and theories to explain his ...
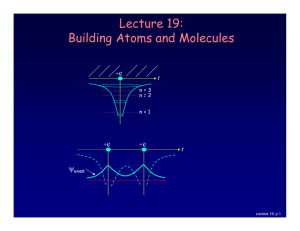
Lecture 19: Building Atoms and Molecules
... Let’s start building more complicated atoms to study the Periodic Table. For atoms with many electrons (e.g., carbon: 6, iron: 26, etc.) 4 What energies do the electrons have? “Pauli Exclusion Principle” (1925) No two electrons can be in the same quantum state. For example, in a given atom they cann ...
... Let’s start building more complicated atoms to study the Periodic Table. For atoms with many electrons (e.g., carbon: 6, iron: 26, etc.) 4 What energies do the electrons have? “Pauli Exclusion Principle” (1925) No two electrons can be in the same quantum state. For example, in a given atom they cann ...
General CHemistry Unit 2 Homework Notes
... Subscripts in a chemical formula represent the relative number of each type of atom. The subscript always follows the symbol for the element. Example: In a water molecule, H2O, there are 2 hydrogen atoms and one oxygen atom. Parentheses are used when a subscript affects a group of atoms. Example: th ...
... Subscripts in a chemical formula represent the relative number of each type of atom. The subscript always follows the symbol for the element. Example: In a water molecule, H2O, there are 2 hydrogen atoms and one oxygen atom. Parentheses are used when a subscript affects a group of atoms. Example: th ...
AtomsFirst2e_day6_sec3.7
... •Given a set of quantum numbers, be able to describe the energy level, subshell (s, p, d, or f), and spin state for an electron •Given information about the principle energy level or shell, subshell (s, p, d, or f), and orbital, be able to determine a set of 4 possible quantum numbers for an electro ...
... •Given a set of quantum numbers, be able to describe the energy level, subshell (s, p, d, or f), and spin state for an electron •Given information about the principle energy level or shell, subshell (s, p, d, or f), and orbital, be able to determine a set of 4 possible quantum numbers for an electro ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.