Vocabulary:
... Bohr’s Atomic Model Planetary System Model – Electrons move around the nucleus of an atom, like the planets around the sun. James Maxwell – Proposed that visible light consists of electromagnetic waves. Maxwell Planck – Suggested that atoms and molecules emit energy in discrete quantities, called qu ...
... Bohr’s Atomic Model Planetary System Model – Electrons move around the nucleus of an atom, like the planets around the sun. James Maxwell – Proposed that visible light consists of electromagnetic waves. Maxwell Planck – Suggested that atoms and molecules emit energy in discrete quantities, called qu ...
CHEMISTRY VOCABULARY
... metals lose electrons and non metals gain them to get full shells. COVALENT COMPOUNDS are formed between non metals, bonds contain shared pairs of electrons. If you know something about SALT (sodium chloride) you know something about IONIC COMPOUNDS IONIC COMPOUNDS are like salt, crystalline solids, ...
... metals lose electrons and non metals gain them to get full shells. COVALENT COMPOUNDS are formed between non metals, bonds contain shared pairs of electrons. If you know something about SALT (sodium chloride) you know something about IONIC COMPOUNDS IONIC COMPOUNDS are like salt, crystalline solids, ...
The format of this test is MULTIPLE CHOICE
... 2. Which unit(s) of measurement are usually dependent variables? Which are most often independent variables? Time is an independent variable types of measurements are usually independent varaiables. 3. Label each kind of graph shown and answer the following questions about the graphs ...
... 2. Which unit(s) of measurement are usually dependent variables? Which are most often independent variables? Time is an independent variable types of measurements are usually independent varaiables. 3. Label each kind of graph shown and answer the following questions about the graphs ...
Dispersion of electromagnetic waves in simple dielectrics “Dispersion” means that optical
... A classical model for frequency dependence The electrons experience a harmonic force, F = −e E(x,t) ∝ exp {−i ωt } this x is the electron position So, the equation of motion for an electron is m x’’ = −k x − γ x’ − e E0 exp{ -i ωt } restoring force; the electron is bound in an atom ...
... A classical model for frequency dependence The electrons experience a harmonic force, F = −e E(x,t) ∝ exp {−i ωt } this x is the electron position So, the equation of motion for an electron is m x’’ = −k x − γ x’ − e E0 exp{ -i ωt } restoring force; the electron is bound in an atom ...
Name - Net Start Class
... 29. If one variable increases while the other variable decreases, what type of relationship is it? Sketch a graph of this relationship. An inversely proportional relationship ...
... 29. If one variable increases while the other variable decreases, what type of relationship is it? Sketch a graph of this relationship. An inversely proportional relationship ...
Objective A - TuHS Physics Homepage
... number 79, mass of an alpha is 6.644x10−27 kg) (49 nm) closest approach brings it to within 47 femtometers of a Gold nucleus. What is 2. An Alpha particle’s its energy in eV (Atomic number is 79, mass of an alpha is 6.644x10−27 kg, look up femto in your data packet) (4.8 MeV) 3. Chapter 27: 73(4. ...
... number 79, mass of an alpha is 6.644x10−27 kg) (49 nm) closest approach brings it to within 47 femtometers of a Gold nucleus. What is 2. An Alpha particle’s its energy in eV (Atomic number is 79, mass of an alpha is 6.644x10−27 kg, look up femto in your data packet) (4.8 MeV) 3. Chapter 27: 73(4. ...
o Schrödinger equation for o Two-electron atoms. o Multi
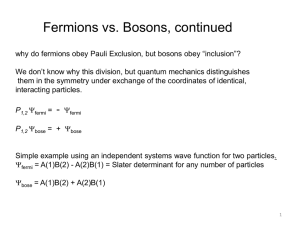
... 1. Parallel spins make the spin part of the wavefunction symmetric. 2. Total wavefunction for electrons must be antisymmetric since electrons are fermions. 3. This forces space part of wavefunction to be antisymmetric. 4. Antisymmetric space wavefunction implies a larger average distance between ...
... 1. Parallel spins make the spin part of the wavefunction symmetric. 2. Total wavefunction for electrons must be antisymmetric since electrons are fermions. 3. This forces space part of wavefunction to be antisymmetric. 4. Antisymmetric space wavefunction implies a larger average distance between ...
Ionic Bonding - cloudfront.net
... To draw Lewis structures for covalent bonds, use the NAS method: N (______________): Find the number of electrons needed to form full octets for all elements involved. For most nonmetals, the number needed is 8. Hydrogen is the ...
... To draw Lewis structures for covalent bonds, use the NAS method: N (______________): Find the number of electrons needed to form full octets for all elements involved. For most nonmetals, the number needed is 8. Hydrogen is the ...
CHE 106 Chapter 6
... Pauli Exclusion Principle: No two electrons can have the same set of four quantum numbers n,l, ml and ms. In order to put electrons in the same orbital, they must have different ms numbers = opposite spins. ...
... Pauli Exclusion Principle: No two electrons can have the same set of four quantum numbers n,l, ml and ms. In order to put electrons in the same orbital, they must have different ms numbers = opposite spins. ...
File - Mr. Holz`s Website
... 15. Know the 4 main properties of enzymes: a. They are proteins b. They bind to specific substrates at the ACTIVE SITE like a lock and key c. Enzymes remain unchanged after a reaction, so they can continue doing their job (1 enzyme can bind to one substrate after another after another) d. Enzymes ca ...
... 15. Know the 4 main properties of enzymes: a. They are proteins b. They bind to specific substrates at the ACTIVE SITE like a lock and key c. Enzymes remain unchanged after a reaction, so they can continue doing their job (1 enzyme can bind to one substrate after another after another) d. Enzymes ca ...
File - docstover.org
... Write out the electron distribution for the elementPhosphorus according to Hund’s rule. Use arrows to represent electrons. 1s2 ____ 2s2 _____ 2p6 _____ _____ ____ 3s2 _____ 3p6 _____ _____ _____ Write the electron configuration for the following elements: Calcium Iodine Vandium Emission (or brig ...
... Write out the electron distribution for the elementPhosphorus according to Hund’s rule. Use arrows to represent electrons. 1s2 ____ 2s2 _____ 2p6 _____ _____ ____ 3s2 _____ 3p6 _____ _____ _____ Write the electron configuration for the following elements: Calcium Iodine Vandium Emission (or brig ...
GENERAL CHEMISTRY SECTION I: ATOMIC THEORY
... For any general chemistry course taught with an “atoms up” approach, an overview of the first chapter requires an argument for how light interacting with matter paved the way for a quantum mechanical revolution, which ultimately revealed the electronic configuration of atoms. Here is a summary of th ...
... For any general chemistry course taught with an “atoms up” approach, an overview of the first chapter requires an argument for how light interacting with matter paved the way for a quantum mechanical revolution, which ultimately revealed the electronic configuration of atoms. Here is a summary of th ...
Name Date Class Period ______
... Matching: Using the word box below, write the letter of the correct vocabulary word for each definition. ...
... Matching: Using the word box below, write the letter of the correct vocabulary word for each definition. ...
Atoms1 - Cbsephysicstutorials
... d) The electrons revolve around the nucleus in various orbits just as planets revolve around the sun. e) The centripetal force required for their revolution is provided by the electrostatic attraction between the electrons and the nucleus. • Draw-back of Rutherford Model: This model could not explai ...
... d) The electrons revolve around the nucleus in various orbits just as planets revolve around the sun. e) The centripetal force required for their revolution is provided by the electrostatic attraction between the electrons and the nucleus. • Draw-back of Rutherford Model: This model could not explai ...
The Pauli exclusion principle states that no two fermions
... the way atoms share electrons. It explains the variety of chemical elements and their ...
... the way atoms share electrons. It explains the variety of chemical elements and their ...
CHEM1405 2012-J-2 June 2012 • What is the ground state electron
... respectively. What is the definition of half-life? Half-life is the amount of time required for the amount (or activity) of a sample to decrease to half its initial value. What percentage of both isotopes will still be detectable after 25 years? The number of nuclei, N, decays with time, t, accordin ...
... respectively. What is the definition of half-life? Half-life is the amount of time required for the amount (or activity) of a sample to decrease to half its initial value. What percentage of both isotopes will still be detectable after 25 years? The number of nuclei, N, decays with time, t, accordin ...
Handout 1: A More Detailed Look at Electronic Structure.
... Spin and orbital angular momenta can interact (couple) with one another and states which have different values for the combined angular momentum will differ in energy. Ligand electrical fields can restrict the motion of electrons about the nucleus thus quenching the orbital angular momentum and grea ...
... Spin and orbital angular momenta can interact (couple) with one another and states which have different values for the combined angular momentum will differ in energy. Ligand electrical fields can restrict the motion of electrons about the nucleus thus quenching the orbital angular momentum and grea ...
Chemistry - Halifax County Public Schools
... The mass of zinc used and hydrogen production are directly proportional. The mass of zinc used and hydrogen production are inversely proportional. The mass of zinc used and hydrogen production are related, but not proportional. The mass of zinc used and hydrogen production are unrelated. ...
... The mass of zinc used and hydrogen production are directly proportional. The mass of zinc used and hydrogen production are inversely proportional. The mass of zinc used and hydrogen production are related, but not proportional. The mass of zinc used and hydrogen production are unrelated. ...
Chem 115 POGIL Worksheet - Week 10 Periodic Trends Why? The
... 2. The experimentally determined Bi–Cl bond distance in bismuth trichloride is 2.48 Å. Given the tabulated value of 0.99 Å for the atomic radius of Cl, predict the atomic radius of Bi. If the experimentally determined Bi–I distance in bismuth triiodide is 2.81 Å, predict the atomic radius of I. How ...
... 2. The experimentally determined Bi–Cl bond distance in bismuth trichloride is 2.48 Å. Given the tabulated value of 0.99 Å for the atomic radius of Cl, predict the atomic radius of Bi. If the experimentally determined Bi–I distance in bismuth triiodide is 2.81 Å, predict the atomic radius of I. How ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.