Lecture 4
... Now, we need to include spin in our description. Electrons have spin antisymmetric. ...
... Now, we need to include spin in our description. Electrons have spin antisymmetric. ...
Lecture
... Hund’s rules - determine the energy levels for the same configuration (generally correct, but not absolutely right) (i) Among all the terms derived from the same configuration, those with the highest spin multiplicity are the lowest in energy. (ii) Of the terms with the same multiplicity, the lowes ...
... Hund’s rules - determine the energy levels for the same configuration (generally correct, but not absolutely right) (i) Among all the terms derived from the same configuration, those with the highest spin multiplicity are the lowest in energy. (ii) Of the terms with the same multiplicity, the lowes ...
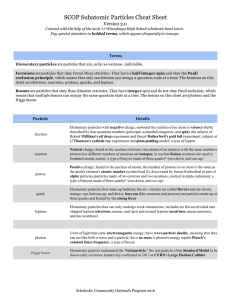
SCOP Subatomic Particles Cheat Sheet
... Fermions are particles that obey FermiDirac statistics. They have a halfinteger spin and obey the Pauli exclusion principle , which means that only one fermion can occupy a quantum state at a time. The fermions on this sheet are ...
... Fermions are particles that obey FermiDirac statistics. They have a halfinteger spin and obey the Pauli exclusion principle , which means that only one fermion can occupy a quantum state at a time. The fermions on this sheet are ...
Atomic 1
... Coupling of orbital angular momenta • For two electron case • l1 orbital angular momentum for first electron • l2 orbital angular momentum for second electron • After coupling resultant angular momentum: L = (l1 - l2) to (l1 +l2) ...
... Coupling of orbital angular momenta • For two electron case • l1 orbital angular momentum for first electron • l2 orbital angular momentum for second electron • After coupling resultant angular momentum: L = (l1 - l2) to (l1 +l2) ...
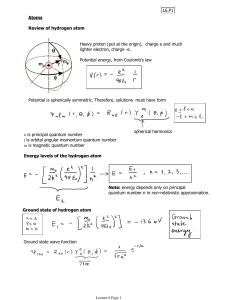
2.4. Quantum Mechanical description of hydrogen atom
... Why is m called the magnetic quantum number? m determines the z component of the angular momentum. Since the electron is moving around the nuclei, and has a charge, it creates magnetic moment. There is a proportional relation between angular momentum and magnetic moment: ...
... Why is m called the magnetic quantum number? m determines the z component of the angular momentum. Since the electron is moving around the nuclei, and has a charge, it creates magnetic moment. There is a proportional relation between angular momentum and magnetic moment: ...
Document
... normally have random spin orientations. In the presence of a strong magnetic field, they become aligned with a component paralell to the field. A brief radio signal flips the spins; as their components reorient paralell to the field, they emit signals that are picked up by sensitive detectors. The d ...
... normally have random spin orientations. In the presence of a strong magnetic field, they become aligned with a component paralell to the field. A brief radio signal flips the spins; as their components reorient paralell to the field, they emit signals that are picked up by sensitive detectors. The d ...
Quantum Numbers Power Point NOTES
... XX – Group # assigned in blocks to each state XXXX – Serial # assigned in blocks to each state SSN = capacity of nearly 1 billion numbers …as of November 1982 = ~ 277 million number issued leaving 75% still available. ...
... XX – Group # assigned in blocks to each state XXXX – Serial # assigned in blocks to each state SSN = capacity of nearly 1 billion numbers …as of November 1982 = ~ 277 million number issued leaving 75% still available. ...
Lecture 12
... If you combine any angular momentum I and J you get every value of angular momentum F according to the rule: ...
... If you combine any angular momentum I and J you get every value of angular momentum F according to the rule: ...
The Weird World of Quantum Information
... the magnetic dipole moments of atoms. The results of these experiments could not be explained by classical mechanics. First, let's discuss why would atom poses a magnetic moment. Even in Bohr's model of the hydrogen atom, an electron, which is a charged particle, occupies a circular orbit, rotating ...
... the magnetic dipole moments of atoms. The results of these experiments could not be explained by classical mechanics. First, let's discuss why would atom poses a magnetic moment. Even in Bohr's model of the hydrogen atom, an electron, which is a charged particle, occupies a circular orbit, rotating ...
EMR_spectra_in_nanoparticles
... Sov. Phys.- JETP 75, 764 (1992); Yu. L. Raikher, V. I. Stepanov, Phys. Rev. B 50, 6250 (1994)] is that individual contributions are taking into account from every quantum transition in the multi-level energy spectrum of the giant spin cluster. Unlike the classical model, this approach explains a nar ...
... Sov. Phys.- JETP 75, 764 (1992); Yu. L. Raikher, V. I. Stepanov, Phys. Rev. B 50, 6250 (1994)] is that individual contributions are taking into account from every quantum transition in the multi-level energy spectrum of the giant spin cluster. Unlike the classical model, this approach explains a nar ...
Chapter 17 - Ferment Magazine
... effect, redistributes all the quantum numbers in a peculiar fashion that is far from being understood. It does however generate a beam of klamps. 3 The mass of the klamp is given by: Mklamp = 6 electrons + one graviton + 1 topological diquark - 2 antiquarks ( 'up' and 'strangeness ) . ...
... effect, redistributes all the quantum numbers in a peculiar fashion that is far from being understood. It does however generate a beam of klamps. 3 The mass of the klamp is given by: Mklamp = 6 electrons + one graviton + 1 topological diquark - 2 antiquarks ( 'up' and 'strangeness ) . ...