1 - shawnschmitt
... d. Dependent variable- the variable that changes as a result of the changes to the independent variable e. Control- a group in which the independent variable is not manipulated, used for comparison f. Hypothesis- a possible explaination for observations, a testable idea g. Mole- the amount of partic ...
... d. Dependent variable- the variable that changes as a result of the changes to the independent variable e. Control- a group in which the independent variable is not manipulated, used for comparison f. Hypothesis- a possible explaination for observations, a testable idea g. Mole- the amount of partic ...
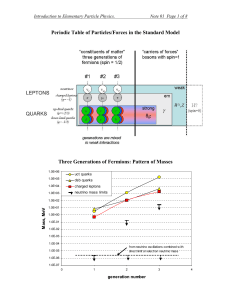
Periodic Table of Particles/Forces in the Standard Model
... There are 4 primary SI units: three kinematical (meter, second, kilogram) and one electrical (Ampere1) It is common in the realm of the elementary particle physics to redefine units so that speed of light and Plank’s constant become equal to one: c=1 and ℏ =1. This imposes two constraints on the thr ...
... There are 4 primary SI units: three kinematical (meter, second, kilogram) and one electrical (Ampere1) It is common in the realm of the elementary particle physics to redefine units so that speed of light and Plank’s constant become equal to one: c=1 and ℏ =1. This imposes two constraints on the thr ...
Transparancies for Atomic Structure Section
... Experiment with silver atoms, 1921, saw some EVEN numbers of lines Non-uniform B field, need a force not just a twist ...
... Experiment with silver atoms, 1921, saw some EVEN numbers of lines Non-uniform B field, need a force not just a twist ...
Light and other electromagnetic radiation – applications in biology
... In 1887 Hertz tested Maxwell's hypothesis. He used an oscillator made of polished brass knobs, each connected to an induction coil and separated by a tiny gap over which sparks could leap. Hertz reasoned that, if Maxwell's predictions were correct, electromagnetic waves would be transmitted during e ...
... In 1887 Hertz tested Maxwell's hypothesis. He used an oscillator made of polished brass knobs, each connected to an induction coil and separated by a tiny gap over which sparks could leap. Hertz reasoned that, if Maxwell's predictions were correct, electromagnetic waves would be transmitted during e ...
Effective Nuclear Charge
... Exchange Particle in CPH Theory As I told before particle charges use color-charges that exist in perimeter produce virtual photons. Electron produces negative virtual photon and proton produces positive virtual photon. So, they put out electricity fields around themselves. Now look at two charge p ...
... Exchange Particle in CPH Theory As I told before particle charges use color-charges that exist in perimeter produce virtual photons. Electron produces negative virtual photon and proton produces positive virtual photon. So, they put out electricity fields around themselves. Now look at two charge p ...
Quantum Numbers, Orbitals, Electron Configurations, Periodic Trends
... Quantum Numbers, Orbitals, Electron Configurations, Periodic Trends CH2000: Introduction to General Chemistry, Plymouth State University, Fall 2014 1. Briefly describe in your own terms what each of the quantum numbers mean: n (principle q.n.) _____________________________________ ℓ (angular momentu ...
... Quantum Numbers, Orbitals, Electron Configurations, Periodic Trends CH2000: Introduction to General Chemistry, Plymouth State University, Fall 2014 1. Briefly describe in your own terms what each of the quantum numbers mean: n (principle q.n.) _____________________________________ ℓ (angular momentu ...
The Quantum Theory of Atoms and Molecules
... 2. The Bohr model treats the electron as if it were a miniature planet, with definite radius and momentum. This is in direct violation of the uncertainty principle which dictates that position and momentum cannot be simultaneously determined. 3. Results were wrong even for atoms with two electrons – ...
... 2. The Bohr model treats the electron as if it were a miniature planet, with definite radius and momentum. This is in direct violation of the uncertainty principle which dictates that position and momentum cannot be simultaneously determined. 3. Results were wrong even for atoms with two electrons – ...
HW-1-Ch1-Atomic-structure-W16
... 4. Calculate the binding energy per nucleon (MeV) of 56Fe isotope of mass 55.952918 amu. ( P= 1.007277 amu,; N= 1.008665 amu; e- = 5.486 x10-4 amu) ...
... 4. Calculate the binding energy per nucleon (MeV) of 56Fe isotope of mass 55.952918 amu. ( P= 1.007277 amu,; N= 1.008665 amu; e- = 5.486 x10-4 amu) ...
Welcome to Physics 112N
... blackbody spectrum only at long wavelengths. At short wavelengths there is complete disagreement. This disagreement between observations and the classical theory is known as the ultraviolet catastrophe. ...
... blackbody spectrum only at long wavelengths. At short wavelengths there is complete disagreement. This disagreement between observations and the classical theory is known as the ultraviolet catastrophe. ...
Unit 4 review sheet
... 36. Heisenberg stated that, at the same time, it was impossible to know what two things about the electron? 37. How many quantum numbers are there? 38. What letter denotes the quantum number for the principle energy level? 39. What four letters are used to represent the sublevels within a principle ...
... 36. Heisenberg stated that, at the same time, it was impossible to know what two things about the electron? 37. How many quantum numbers are there? 38. What letter denotes the quantum number for the principle energy level? 39. What four letters are used to represent the sublevels within a principle ...
BWilliamsLtalk - FSU High Energy Physics
... Young in 1803, using a modified version of the double-slit experiment ...
... Young in 1803, using a modified version of the double-slit experiment ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.