Define:
... 50. Where are the electrons and the protons in the Bohr model? 51. The principal quantum number indicates the _____ _____ of an electron. 52. What is the shape of the following orbitals: s, p 53. How are frequency and wavelength related? 54. What causes the emission of light from an atom? 55. All at ...
... 50. Where are the electrons and the protons in the Bohr model? 51. The principal quantum number indicates the _____ _____ of an electron. 52. What is the shape of the following orbitals: s, p 53. How are frequency and wavelength related? 54. What causes the emission of light from an atom? 55. All at ...
Charges Near Magnets—Magnetic Force184 The figures below
... The figures below show electrically charged particles sitting at rest near the poles of permanent magnets. All of the permanent magnets are the same strength. The magnitudes and signs of the electric charges are shown in the circles, which represent the particles. The zero values indicate that the p ...
... The figures below show electrically charged particles sitting at rest near the poles of permanent magnets. All of the permanent magnets are the same strength. The magnitudes and signs of the electric charges are shown in the circles, which represent the particles. The zero values indicate that the p ...
+ e - Indico
... to call neutrons, which have spin ½ and obey the exclusion principle ..... The mass of the neutrons should be of the same order of magnitude as the electron mass and in any event not larger than 0.01 proton masses. The continuous -spectrum would then become understandable by the assumption that in ...
... to call neutrons, which have spin ½ and obey the exclusion principle ..... The mass of the neutrons should be of the same order of magnitude as the electron mass and in any event not larger than 0.01 proton masses. The continuous -spectrum would then become understandable by the assumption that in ...
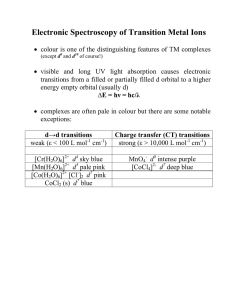
Electronic Spectroscopy of Transition Metal Ions
... • combine these into S and L which represent the TOTAL spin and orbital angular momentum • there are many different arrangements of electrons in d orbitals so this gives rise to many possible states (RussellSaunders ‘terms’) that represent different energies for the system as a whole eg. d2 ion 1st ...
... • combine these into S and L which represent the TOTAL spin and orbital angular momentum • there are many different arrangements of electrons in d orbitals so this gives rise to many possible states (RussellSaunders ‘terms’) that represent different energies for the system as a whole eg. d2 ion 1st ...
Glossary of Key Terms in Chapter Two
... mass number (2.1 ) the sum of the number of protons and neutrons in an atom. metal (2.4) element located on the left side of the periodic table (left of the “staircase” boundary). metalloid (2.4) an element along the “staircase” boundary between metals and nonmetals; they exhibit both metallic and n ...
... mass number (2.1 ) the sum of the number of protons and neutrons in an atom. metal (2.4) element located on the left side of the periodic table (left of the “staircase” boundary). metalloid (2.4) an element along the “staircase” boundary between metals and nonmetals; they exhibit both metallic and n ...
Cyclic Reactor_Patent Application_1
... combined three beams (three unidirectional currents) will compress the whole system in radial direction (pinch-effect). In fact pinch-effect will be provided thanks to the circumstance that in frame of reference connected with ions combined beam will charged negatively and for frame of reference con ...
... combined three beams (three unidirectional currents) will compress the whole system in radial direction (pinch-effect). In fact pinch-effect will be provided thanks to the circumstance that in frame of reference connected with ions combined beam will charged negatively and for frame of reference con ...
Periodic Trends - cloudfront.net
... their atomic numbers; properties of the elements occurred at repeated intervals called periods. This defines the property of periodicity Periodic Trends-properties that show patterns when examined across the periods or vertically down the groups while there are many periodic trends, we will focu ...
... their atomic numbers; properties of the elements occurred at repeated intervals called periods. This defines the property of periodicity Periodic Trends-properties that show patterns when examined across the periods or vertically down the groups while there are many periodic trends, we will focu ...
Frank-Hertz Experiment with Argon
... grid G2 is set up at a somewhat greater distance, and the collector electrode P is set up next to it. The cathode is heated indirectly, in Fig. 1: Schematic diagram of the Franck-Hertz tube, Ar order to prevent a potential differential along K. Electrons are emitted by the hot electrode and form a c ...
... grid G2 is set up at a somewhat greater distance, and the collector electrode P is set up next to it. The cathode is heated indirectly, in Fig. 1: Schematic diagram of the Franck-Hertz tube, Ar order to prevent a potential differential along K. Electrons are emitted by the hot electrode and form a c ...
E - Purdue Physics
... • external photon causes electron jump to lower level • a photon is emitted • the original photon is not ...
... • external photon causes electron jump to lower level • a photon is emitted • the original photon is not ...
Electron Structure of Atoms Notes
... (mass = 9.11 x10 -31 kg) traveling at a speed of 1.0 x107 m/s with that of a ball (mass = 0.10 kg) traveling at 35 m/s ...
... (mass = 9.11 x10 -31 kg) traveling at a speed of 1.0 x107 m/s with that of a ball (mass = 0.10 kg) traveling at 35 m/s ...
2 The interaction of energetic particles with material
... This is another interaction between an energetic charged particle and material. The particle interacts with a bounded charged particle in material, and as a result a gamma (photon) is being created. The energy of the emitted photon and the (continuing) particle equals the original energy op the inco ...
... This is another interaction between an energetic charged particle and material. The particle interacts with a bounded charged particle in material, and as a result a gamma (photon) is being created. The energy of the emitted photon and the (continuing) particle equals the original energy op the inco ...
SESSION 6: ELECTROMAGNETIC RADIATION KEY CONCEPTS: X
... Wave-like nature of EM radiation Photon: A photon is a quantum (energy packet) of light Fields: Wave equation is still valid: ...
... Wave-like nature of EM radiation Photon: A photon is a quantum (energy packet) of light Fields: Wave equation is still valid: ...
The Quantum Model of the Atom
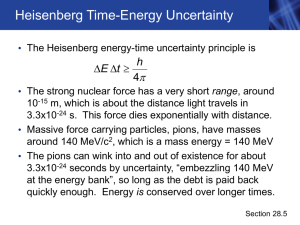
... • Werner Heisenberg • 1927 – German theoretical physicist • Idea involved the detection of electrons, which are detected by their interactions with photons • Because photons have about the same energy as electrons, any attempt to locate a specific electron with a photon knocks the electron off its c ...
... • Werner Heisenberg • 1927 – German theoretical physicist • Idea involved the detection of electrons, which are detected by their interactions with photons • Because photons have about the same energy as electrons, any attempt to locate a specific electron with a photon knocks the electron off its c ...
Essential Question: What is the current model of the atom? How
... Draw a picture of what we think the atom looks like today: ...
... Draw a picture of what we think the atom looks like today: ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.