study note 1 06
... matter that predicts quantized energy levels). What two kinds of waves exist? Standing and traveling. Give an example of a standing wave. Guitar string, electron. What are the different parts of a wave? The part that oscillates between crest and trough and nodes (fixed). What is the restriction plac ...
... matter that predicts quantized energy levels). What two kinds of waves exist? Standing and traveling. Give an example of a standing wave. Guitar string, electron. What are the different parts of a wave? The part that oscillates between crest and trough and nodes (fixed). What is the restriction plac ...
Chapter 4 Key Terms - Lower Cape May Regional School District
... atomic number - the number of protons in the nucleus of an atom average atomic mass - the weighted average of the masses of all naturally occurring isotopes of an element mass number - the total number of protons and neutrons in the nucleus of an atom isotopes - any atoms having the same number of p ...
... atomic number - the number of protons in the nucleus of an atom average atomic mass - the weighted average of the masses of all naturally occurring isotopes of an element mass number - the total number of protons and neutrons in the nucleus of an atom isotopes - any atoms having the same number of p ...
HChemTROCh17Sec3PositronsAND10Exposure
... Background Radiation • Radiation from natural sources including: ▫ Cosmic rays, radioisotopes in the air, water, soil, and rocks ...
... Background Radiation • Radiation from natural sources including: ▫ Cosmic rays, radioisotopes in the air, water, soil, and rocks ...
History of Particle Physics (lecture notes)
... A major advance was Chadwick's discovery of the neutron, in 1932. It is the prototype of a "missing mass" experiment. More here: hyperphysics.phy-‐ astr.gsu.edu/hbase/particles/neutrondis.html. ...
... A major advance was Chadwick's discovery of the neutron, in 1932. It is the prototype of a "missing mass" experiment. More here: hyperphysics.phy-‐ astr.gsu.edu/hbase/particles/neutrondis.html. ...
Chapter 30
... when α particles, β particles, or y rays strike them. Thus, photographic film can be used to detect these particles and rays. Many other devices are used to detect charged particles and rays. Most of these devices make use of the fact that a collision with a high speed particle will remove electrons ...
... when α particles, β particles, or y rays strike them. Thus, photographic film can be used to detect these particles and rays. Many other devices are used to detect charged particles and rays. Most of these devices make use of the fact that a collision with a high speed particle will remove electrons ...
sch4u-quantumtheory
... Electrons in their lowest possible energy levels are in the ground state Electrons promoted to any level n > 1 are in an excited state Electrons are promoted by absorbing energy e.g., electric discharge, heat, lasers (photons) Electrons in an excited state eventually drop back down to the ground sta ...
... Electrons in their lowest possible energy levels are in the ground state Electrons promoted to any level n > 1 are in an excited state Electrons are promoted by absorbing energy e.g., electric discharge, heat, lasers (photons) Electrons in an excited state eventually drop back down to the ground sta ...
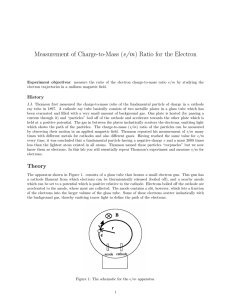
Measurement of Charge-to-Mass (e/m) Ratio for the Electron
... collide with helium atoms, which are excited and then radiate visible light. The electron gun is shown in Fig. 2b. The heater heats the cathode, which emits electrons. The electrons are accelerated by a potential applied between the cathode and the anode. The grid is held positive with respect to th ...
... collide with helium atoms, which are excited and then radiate visible light. The electron gun is shown in Fig. 2b. The heater heats the cathode, which emits electrons. The electrons are accelerated by a potential applied between the cathode and the anode. The grid is held positive with respect to th ...
Chap. 13 -- Atomic P..
... 5. Identify the electronic transition (initial energy level final energy level) corresponding to each of the four listed photons: a) H b) H c) H d) H (These four are said to be part of the “Balmer” series.) 6. Is it possible for an electron in a hydrogen atom to emit a gamma ray by falling to ...
... 5. Identify the electronic transition (initial energy level final energy level) corresponding to each of the four listed photons: a) H b) H c) H d) H (These four are said to be part of the “Balmer” series.) 6. Is it possible for an electron in a hydrogen atom to emit a gamma ray by falling to ...
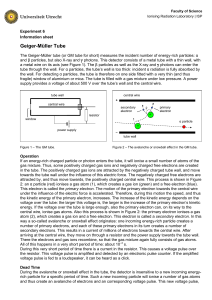
6 Geiger-Müller Tube - Ioniserende Stralen Practicum
... gas mixture. Thus, some positively charged gas ions and negatively charged free electrons are created in the tube. The positively charged gas ions are attracted by the negatively charged tube wall, and move towards the tube wall under the influence of this electric force. The negatively charged free ...
... gas mixture. Thus, some positively charged gas ions and negatively charged free electrons are created in the tube. The positively charged gas ions are attracted by the negatively charged tube wall, and move towards the tube wall under the influence of this electric force. The negatively charged free ...
6.1.2. Number Representation: States
... Consider a set of complete, orthonormal 1-particle (1-P) basis. For the sake of clarity, we shall assume the quantum numbers to be discrete. (Results for the continuous case can be obtained by some limiting procedure). To begin, we arrange the 1-P states by some rule into a unique sequence 0,1,2 ...
... Consider a set of complete, orthonormal 1-particle (1-P) basis. For the sake of clarity, we shall assume the quantum numbers to be discrete. (Results for the continuous case can be obtained by some limiting procedure). To begin, we arrange the 1-P states by some rule into a unique sequence 0,1,2 ...
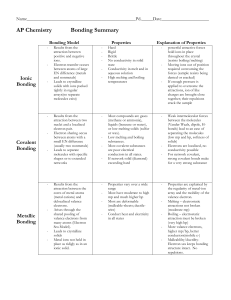
Types of Bonding Summary
... forces (sample resists being dented or cracked) If enough pressure is applied to overcome the attractions, ion of like charges are brought close together; their repulsions crack the sample Weak intermolecular forces between the molecules (Vander Waals, dipole, Hbonds) lead to an ease of separating t ...
... forces (sample resists being dented or cracked) If enough pressure is applied to overcome the attractions, ion of like charges are brought close together; their repulsions crack the sample Weak intermolecular forces between the molecules (Vander Waals, dipole, Hbonds) lead to an ease of separating t ...
SG2 Atoms and Atomic Structure
... e) Use periodic table to determine the number of protons, neutrons, and electrons in a neutral atom f) Discovery of isotopes and what they are g) Calculate average atomic mass of an element from mass number & fractional abundance of isotopes. h) Estimate fractional abundances of isotopes, given thei ...
... e) Use periodic table to determine the number of protons, neutrons, and electrons in a neutral atom f) Discovery of isotopes and what they are g) Calculate average atomic mass of an element from mass number & fractional abundance of isotopes. h) Estimate fractional abundances of isotopes, given thei ...
Ch. 19- Acids and Bases
... • Discoverer of wave-particle duality • De Broglie • Go back to game ...
... • Discoverer of wave-particle duality • De Broglie • Go back to game ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.