CALCULATION OF THE ELECTRON MOBILITY OF GaN
... provides a useful tool for the development, analysis, and understanding of semiconductor devices. The Monte Carlo method in which the Boltzmann equation is not directly solved, but the distribution function and the transport coefficients are evaluated by the simulation of electron trajectories using ...
... provides a useful tool for the development, analysis, and understanding of semiconductor devices. The Monte Carlo method in which the Boltzmann equation is not directly solved, but the distribution function and the transport coefficients are evaluated by the simulation of electron trajectories using ...
UNIT PLAN TEMPLATE
... Given the identity of a nuclide, determine its number of protons, neutrons, and electrons. Explain the mathematical relationship among the speed, wavelength, and frequency, of electromagnetic radiation. Discuss the dual wave-particle nature of light. Describe the Bohr model of the hydrogen atom. Com ...
... Given the identity of a nuclide, determine its number of protons, neutrons, and electrons. Explain the mathematical relationship among the speed, wavelength, and frequency, of electromagnetic radiation. Discuss the dual wave-particle nature of light. Describe the Bohr model of the hydrogen atom. Com ...
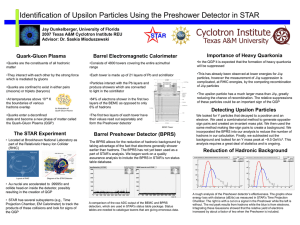
Dr. Saskia Mioduszewski Quark-Gluon Plasma
... • Au nuclei are accelerated to .99995c and collide head-on inside the detector, possibly resulting in the creation of QGP • STAR has several subsystems (e.g., Time Projection Chamber, EM Calorimeter) to track the products of these collisions and look for signs of the QGP ...
... • Au nuclei are accelerated to .99995c and collide head-on inside the detector, possibly resulting in the creation of QGP • STAR has several subsystems (e.g., Time Projection Chamber, EM Calorimeter) to track the products of these collisions and look for signs of the QGP ...
Read Notes #1 - Faculty Website Listing
... A single wave of wavelength, , has a definite momentum, but know specific location. Thus, it can’t represent an object with a definite location. We can form a wave packet by adding many waves with different wavelengths to create an object which is localized at a definite point in space. However, ou ...
... A single wave of wavelength, , has a definite momentum, but know specific location. Thus, it can’t represent an object with a definite location. We can form a wave packet by adding many waves with different wavelengths to create an object which is localized at a definite point in space. However, ou ...
topic 1 sol review homework
... 2SO2(g) + O2(g) 2SO3(g) + heat, which change will shift the equilibrium to the right? a) adding a catalyst b) adding more O2(g) c) decreasing pressure d) increasing temperature 8. Name two reasons why the positively charged alpha particles used by Rutherford were deflected by the nucleus of the g ...
... 2SO2(g) + O2(g) 2SO3(g) + heat, which change will shift the equilibrium to the right? a) adding a catalyst b) adding more O2(g) c) decreasing pressure d) increasing temperature 8. Name two reasons why the positively charged alpha particles used by Rutherford were deflected by the nucleus of the g ...
The “classically forbidden regions” are where … a. a particle`s total
... • You can put them in another energy state (takes more energy) • You can instead change their internal quantum numbers (if they have them). • Electrons do have 1 internal number that can be +1/2 or -1/2 so 2 of them can get into a state. ...
... • You can put them in another energy state (takes more energy) • You can instead change their internal quantum numbers (if they have them). • Electrons do have 1 internal number that can be +1/2 or -1/2 so 2 of them can get into a state. ...
Webquest: Dividing the Indivisible Use the following web sites and
... electron, the nucleus, the proton, and the neutron. These discoveries happened over a 35year period and each discovery had a huge impact on our understanding of atoms. Suggested Web Resources: • A Look Inside the Atom • Rutherford and the Atomic Nucleus • Chadwick Discovers the Neutron As you comple ...
... electron, the nucleus, the proton, and the neutron. These discoveries happened over a 35year period and each discovery had a huge impact on our understanding of atoms. Suggested Web Resources: • A Look Inside the Atom • Rutherford and the Atomic Nucleus • Chadwick Discovers the Neutron As you comple ...
Radiation in a Medium
... • All atmospheric processes are driven by solar energy. Radiative transfer explains how this energy is distributed throughout the atmosphere. • The vast majority of our information concerning the atmospheric state is derived from radiation measurements. These measurements cannot be properly interpre ...
... • All atmospheric processes are driven by solar energy. Radiative transfer explains how this energy is distributed throughout the atmosphere. • The vast majority of our information concerning the atmospheric state is derived from radiation measurements. These measurements cannot be properly interpre ...
Atomic Physics
... States with same n, have same energy and can have " = 0,1,2,...,n-1 orbital quantum number " =0 orbits are most elliptical " =n-1 most circular The z component of the angular momentum must also be quantized ...
... States with same n, have same energy and can have " = 0,1,2,...,n-1 orbital quantum number " =0 orbits are most elliptical " =n-1 most circular The z component of the angular momentum must also be quantized ...
lhc
... conditions that existed a fraction of a second after the birth of the Universe. It is the biggest, and also the most expensive, such machine built to view and understand the smallest fragments of matter in the universe. It is indeed the world's biggest microscope. The milestone was achieved on March ...
... conditions that existed a fraction of a second after the birth of the Universe. It is the biggest, and also the most expensive, such machine built to view and understand the smallest fragments of matter in the universe. It is indeed the world's biggest microscope. The milestone was achieved on March ...
Quantum Statistics Applications
... • # of available states (“nodes”) for any wavelength • wavelength --> momentum --> energy • “standing wave” counting often holds:often called “gas” but can be solid/liquid. Solve Scrd. Eq. In 1D d2 dx 2 ...
... • # of available states (“nodes”) for any wavelength • wavelength --> momentum --> energy • “standing wave” counting often holds:often called “gas” but can be solid/liquid. Solve Scrd. Eq. In 1D d2 dx 2 ...
75 Years of Particle Accelerators
... First treatment by the Lawrence's of their mother. Stone in the late 30’ and neutrons. (Sad story) Linacs for x-rays built by Siemans and Varian in the US Hadron therapy (Bragg peak) suggested by Bob Wilson in 1946. Pioneered in Berkeley and Harvard. Now 5 facilities in US; many more to come. Heavy ...
... First treatment by the Lawrence's of their mother. Stone in the late 30’ and neutrons. (Sad story) Linacs for x-rays built by Siemans and Varian in the US Hadron therapy (Bragg peak) suggested by Bob Wilson in 1946. Pioneered in Berkeley and Harvard. Now 5 facilities in US; many more to come. Heavy ...
File
... emission spectrum consists of discrete wavelength rather than a continuum wavelength? (b) Account for the existence of several series of lines in the spectrum. What quantity distinguishes one series of lines from another? (c) Draw an electronic energy level diagram for the hydrogen atom and indicate ...
... emission spectrum consists of discrete wavelength rather than a continuum wavelength? (b) Account for the existence of several series of lines in the spectrum. What quantity distinguishes one series of lines from another? (c) Draw an electronic energy level diagram for the hydrogen atom and indicate ...
Introduction to Quantum Mechanics Notes
... the rent we pay for our room on Earth.” -Sir Wilfred Grenfell 1.What does this mean to you? 2.How can you be of service to others? ...
... the rent we pay for our room on Earth.” -Sir Wilfred Grenfell 1.What does this mean to you? 2.How can you be of service to others? ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.