No Slide Title
... Time duration of the scattered photons For head configuration the pulse duration will be determined by either the electron bunch length if Lb
... Time duration of the scattered photons For head configuration the pulse duration will be determined by either the electron bunch length if Lb
Chapter 7 Radiation from Charged Particle Interaction with Matter
... mass, however, is a very important effect. Light projectiles like electrons or positrons are far more efficient radiaters (by the inverse mass ratio) than protons or heavy nuclei, because their acceleration is so much greater. That said, however, we realize that if a heavy projectile is colliding with ...
... mass, however, is a very important effect. Light projectiles like electrons or positrons are far more efficient radiaters (by the inverse mass ratio) than protons or heavy nuclei, because their acceleration is so much greater. That said, however, we realize that if a heavy projectile is colliding with ...
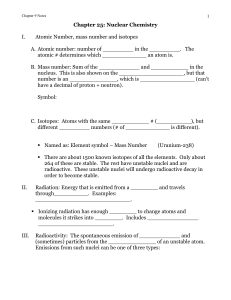
Chapter 9: Nuclear Chemistry
... left side of the arrow must equal the sum of the _________________ on the right. Same rules apply to the atomic numbers. B. Example: decay of U-238 C. Example: decay of Mg-27 (both positron and electron) ...
... left side of the arrow must equal the sum of the _________________ on the right. Same rules apply to the atomic numbers. B. Example: decay of U-238 C. Example: decay of Mg-27 (both positron and electron) ...
South Pasadena • AP Chemistry Name
... The ionization energy of an element is the energy required to remove the most loosely held electron from atoms of the element in the gaseous state (cf. page 357). It is usually expressed in units of kJ/mol. Given that R, the Rydberg constant, is 1.097 x 107 m-1 h, Planck’s constant, is 6.626 x 10-34 ...
... The ionization energy of an element is the energy required to remove the most loosely held electron from atoms of the element in the gaseous state (cf. page 357). It is usually expressed in units of kJ/mol. Given that R, the Rydberg constant, is 1.097 x 107 m-1 h, Planck’s constant, is 6.626 x 10-34 ...
Ch. 4-2 PowerPoint
... Louis De Broglie stated that electrons could be considered waves confined to a space around an atomic nucleus. ...
... Louis De Broglie stated that electrons could be considered waves confined to a space around an atomic nucleus. ...
Chapter Summary
... In our modern view of the atom, the nucleus is modeled as a point particle with a positive charge. The chemical properties of the atom are dominated by the behavior of the atom’s electrons. In contrast to the popular representation of an atom being made up of a positive nucleus orbited by electrons ...
... In our modern view of the atom, the nucleus is modeled as a point particle with a positive charge. The chemical properties of the atom are dominated by the behavior of the atom’s electrons. In contrast to the popular representation of an atom being made up of a positive nucleus orbited by electrons ...
Standard EPS Shell Presentation
... of the electrons as moving around the nucleus in an area called an electron cloud. The energy levels occur because electrons in the cloud are at different average distances from the nucleus. ...
... of the electrons as moving around the nucleus in an area called an electron cloud. The energy levels occur because electrons in the cloud are at different average distances from the nucleus. ...
Atomic Structure
... Cathode ray tubes pass electricity through a gas that is contained at a very low pressure. ...
... Cathode ray tubes pass electricity through a gas that is contained at a very low pressure. ...
Ununpentium does not occur naturally in the Earth`s crust. Following
... name and symbol [678]. It was announced June 2016 that the name moscovium and symbol Mc were proposed. The name is in recognition of the Moscow region and honors the ancient Russian land that is the home of the Joint Institute for Nuclear Research, where the discovery experiments were conducted usin ...
... name and symbol [678]. It was announced June 2016 that the name moscovium and symbol Mc were proposed. The name is in recognition of the Moscow region and honors the ancient Russian land that is the home of the Joint Institute for Nuclear Research, where the discovery experiments were conducted usin ...
Part IV
... Given an energy band ε(k) (a Schrödinger Equation eigenvalue): The Electron is a Quantum Mechanical Wave • From Quantum Mechanics, the energy ε(k) & the frequency ω(k) are related by: ε(k) ħω(k) (1) • Now, from Classical Wave Theory, the wave group velocity v(k) is defined as: ...
... Given an energy band ε(k) (a Schrödinger Equation eigenvalue): The Electron is a Quantum Mechanical Wave • From Quantum Mechanics, the energy ε(k) & the frequency ω(k) are related by: ε(k) ħω(k) (1) • Now, from Classical Wave Theory, the wave group velocity v(k) is defined as: ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 17. Ψ = (2a/π)1/4 exp(-ax2) is an eigen function of the Hamiltonian operator for the 1D SHO. Find the eigenvalue E and express it in terms of the classical frequency ν, where ν = (1/2π)√(k/m) and the constant a = (π/h)√(km). 18. What is an atomic term symbol? Write the term symbols for the excited ...
... 17. Ψ = (2a/π)1/4 exp(-ax2) is an eigen function of the Hamiltonian operator for the 1D SHO. Find the eigenvalue E and express it in terms of the classical frequency ν, where ν = (1/2π)√(k/m) and the constant a = (π/h)√(km). 18. What is an atomic term symbol? Write the term symbols for the excited ...
3. atomic structure
... in order to excite the electrons to jump from a lower energy level to a higher energy level. When an electron returns from a higher energy state to a lower energy state, it emits a specific amount of energy usually in the form of light. This is known as a bright line spectrum, and can be used to ide ...
... in order to excite the electrons to jump from a lower energy level to a higher energy level. When an electron returns from a higher energy state to a lower energy state, it emits a specific amount of energy usually in the form of light. This is known as a bright line spectrum, and can be used to ide ...
Quantum Theory and Atomic Structure
... • Heisenberg’s uncertainty principle (1927) – the exact position and momentum (velocity) of a particle can not be known simultaneously ∆x⋅∆p ≥ h/4π ∆x and ∆p = m∆u – uncertainty in position and momentum, respectively – A consequence of the wave-particle duality of matter – The exact location of very ...
... • Heisenberg’s uncertainty principle (1927) – the exact position and momentum (velocity) of a particle can not be known simultaneously ∆x⋅∆p ≥ h/4π ∆x and ∆p = m∆u – uncertainty in position and momentum, respectively – A consequence of the wave-particle duality of matter – The exact location of very ...
Particle Physics Experiments
... “relativistic effects” special relativity tells us that certain approximations made in Newtonian mechanics break down at very high speeds; relation between momentum and velocity in “old” (Newtonian) mechanics: p = m v becomes ________ p = mo v , with = 1/1 - (v/c)2 mo = “rest mass”, i.e. mas ...
... “relativistic effects” special relativity tells us that certain approximations made in Newtonian mechanics break down at very high speeds; relation between momentum and velocity in “old” (Newtonian) mechanics: p = m v becomes ________ p = mo v , with = 1/1 - (v/c)2 mo = “rest mass”, i.e. mas ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.