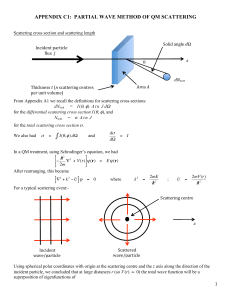
Quantum1
... Given the Uncertainty Principle, how do you write an equation of motion for a particle? •First, remember that a particle is only a particle sort of, and a wave sort of, and it’s not quite like anything you’ve encountered in classical physics. We need to use Fourier’s Theorem to represent the partic ...
... Given the Uncertainty Principle, how do you write an equation of motion for a particle? •First, remember that a particle is only a particle sort of, and a wave sort of, and it’s not quite like anything you’ve encountered in classical physics. We need to use Fourier’s Theorem to represent the partic ...
11 Applications III
... for solids. This is fairly accurate for most solids at room temperature and is known as the law of Dulong and Petit. However, at low temperatures the heat capacity diminishes and a better model is needed. Einstein proposed a quantum mechanical version of the oscillator model and this gave a better d ...
... for solids. This is fairly accurate for most solids at room temperature and is known as the law of Dulong and Petit. However, at low temperatures the heat capacity diminishes and a better model is needed. Einstein proposed a quantum mechanical version of the oscillator model and this gave a better d ...
Modern Physics 3-Atomic Physics
... visible spectral lines produced by hydrogen and represented it with the following equation: ...
... visible spectral lines produced by hydrogen and represented it with the following equation: ...
Periodic Table Notes Unit 3 – Notes
... substances that are poor conductors of heat and electricity. • They tend to gain electrons in reactions with metals to acquire a noble gas configuration. ...
... substances that are poor conductors of heat and electricity. • They tend to gain electrons in reactions with metals to acquire a noble gas configuration. ...
Quarks, Leptons, Bosons the LHC and All That
... Some of What LHC Can Study • Higgs Boson – Understanding M ...
... Some of What LHC Can Study • Higgs Boson – Understanding M ...
The Standard Model - University of Rochester
... and electromagnetic force as two components of one electroweak force ► Predicts W+, W , and Z0 as transmitters of the weak force ► Implies Higgs Boson as a way to give Ws and Z mass ...
... and electromagnetic force as two components of one electroweak force ► Predicts W+, W , and Z0 as transmitters of the weak force ► Implies Higgs Boson as a way to give Ws and Z mass ...
Making predictions In the space below, complete the following table
... Exploration: Exploring Relativistic Effects In this lesson you will use the "Particle in a Magnetic Field" applet to investigate the relativistic behaviour of charged particles moving at high velocity through magnetic fields. ...
... Exploration: Exploring Relativistic Effects In this lesson you will use the "Particle in a Magnetic Field" applet to investigate the relativistic behaviour of charged particles moving at high velocity through magnetic fields. ...
Chapter 5
... Particlelike Properties of Electromagnetic Radiation: The Plank Equation • Blackbody radiation is the visible glow that solid objects emit when heated. • Max Planck (1858–1947): Developed a formula to fit the observations. He proposed that energy is only emitted in discrete packets called quanta. ...
... Particlelike Properties of Electromagnetic Radiation: The Plank Equation • Blackbody radiation is the visible glow that solid objects emit when heated. • Max Planck (1858–1947): Developed a formula to fit the observations. He proposed that energy is only emitted in discrete packets called quanta. ...
The Relativistic Quantum World
... Louis de Broglie - PhD Thesis(!) 1924 (Nobel prize 1929): If light are particles incorporated in a wave, it suggests that particles (electrons) “are carried” by waves. Original idea: a physical wave Quantum mechanics: probability wave! Particle wavelength: ...
... Louis de Broglie - PhD Thesis(!) 1924 (Nobel prize 1929): If light are particles incorporated in a wave, it suggests that particles (electrons) “are carried” by waves. Original idea: a physical wave Quantum mechanics: probability wave! Particle wavelength: ...
Ex4
... Set 4 1. An ideal gas of classical charged particles with mass m is confined between two capacitor plates of area A, separated by distance L. The capacitors produce a force f perpendicular to the plates which pushes the particles to the lower plate. The particles can be absorbed on either plate, wit ...
... Set 4 1. An ideal gas of classical charged particles with mass m is confined between two capacitor plates of area A, separated by distance L. The capacitors produce a force f perpendicular to the plates which pushes the particles to the lower plate. The particles can be absorbed on either plate, wit ...
Chapter 4: The Structure of the Atom &
... 4.2 Subatomic Particles and the Nuclear Atom Subatomic atomic particles o Proton – positive charge _________ 1.673x10-24 g o Neutron – neutral charge ________ About the same size as a proton: 1.675x10-24 g o Electron – negative charge _______ 1/1840 the size of a proton or neutron (9.11x10-2 ...
... 4.2 Subatomic Particles and the Nuclear Atom Subatomic atomic particles o Proton – positive charge _________ 1.673x10-24 g o Neutron – neutral charge ________ About the same size as a proton: 1.675x10-24 g o Electron – negative charge _______ 1/1840 the size of a proton or neutron (9.11x10-2 ...
Chapter 4.2 Notes
... H. Everything scientists know about the ____________________ and _______________________ particles is based on how the particles ________________. We are still ______________ to see the inside of an ________. However, we do have microscope that can show how _____________ are arranged on the ________ ...
... H. Everything scientists know about the ____________________ and _______________________ particles is based on how the particles ________________. We are still ______________ to see the inside of an ________. However, we do have microscope that can show how _____________ are arranged on the ________ ...
Document
... • Proposition: Each electron either goes through slit 1 or slit 2 on its way to the backstop • If so, then for those that pass through slit 1, say, it cannot matter whether slit 2 is open or closed (and vice versa) • The total distribution of electrons at the backstop is thus the sum of those passin ...
... • Proposition: Each electron either goes through slit 1 or slit 2 on its way to the backstop • If so, then for those that pass through slit 1, say, it cannot matter whether slit 2 is open or closed (and vice versa) • The total distribution of electrons at the backstop is thus the sum of those passin ...
January 2001
... Deduce the relation between the magnetic field Bz at radius R and the magnetic field Bz,ave averaged over the area of the circle. Also deduce the maximum energy E to which an electron could be accelerated by a betatron in terms of Bz , dBz,ave /dt and R. Hints: The electrons in this problem are ultr ...
... Deduce the relation between the magnetic field Bz at radius R and the magnetic field Bz,ave averaged over the area of the circle. Also deduce the maximum energy E to which an electron could be accelerated by a betatron in terms of Bz , dBz,ave /dt and R. Hints: The electrons in this problem are ultr ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.