6.5 Synchrotron radiation and damping
... Charged particles radiate when they are deflected in the magnetic field [1] (transverse acceleration). In the ultra-relativistic case, when the particle speed is very close to the speed of light, ≈ c, most of the radiation is emitted in the forward direction [2] into a cone centred on the tangent ...
... Charged particles radiate when they are deflected in the magnetic field [1] (transverse acceleration). In the ultra-relativistic case, when the particle speed is very close to the speed of light, ≈ c, most of the radiation is emitted in the forward direction [2] into a cone centred on the tangent ...
UVM Physics MS: Comprehensive Exam Date: Saturday January 11, 2013 Time:
... (a) Find the electrostatic field everywhere in space. (b) The shell is now rotating around its axis (ẑ-axis) with the frequency ω0 = const. The rotating insulator produces a surface current density. Find the magnetic field generated everywhere in space. (c) After a while the cylinder starts to slow ...
... (a) Find the electrostatic field everywhere in space. (b) The shell is now rotating around its axis (ẑ-axis) with the frequency ω0 = const. The rotating insulator produces a surface current density. Find the magnetic field generated everywhere in space. (c) After a while the cylinder starts to slow ...
Ch. 13 notes
... • Principal Quantum Number (n) = the energy level of the electron: 1, 2, 3, etc. • Within each energy level, the complex math of Schrodinger’s equation describes several shapes. • These are called atomic orbitals (coined by scientists in 1932) - regions where there is a high probability of finding a ...
... • Principal Quantum Number (n) = the energy level of the electron: 1, 2, 3, etc. • Within each energy level, the complex math of Schrodinger’s equation describes several shapes. • These are called atomic orbitals (coined by scientists in 1932) - regions where there is a high probability of finding a ...
QUANTUM MECHANICAL MODEL OF THE ATOM
... have both observable wave like and particle like properties. • Quantum mechanics is based on a fundamental equation which is called Schrodinger equation. • Schrodinger’s equation: For a system (such as an atom or a molecule whose energy does not change with time) the Schrödinger equation is written ...
... have both observable wave like and particle like properties. • Quantum mechanics is based on a fundamental equation which is called Schrodinger equation. • Schrodinger’s equation: For a system (such as an atom or a molecule whose energy does not change with time) the Schrödinger equation is written ...
CTF-3 - CARE-HHH
... this effect would suggest that the total distance travelled through the plasma cannot be more than one or a few radiation lengths for example X0~10 m for 4x1022 e/cm3 using the rough estimate of 30 GV/m for 1x1017e/cm3 this gives an ultimate energy of ~200 TeV ...
... this effect would suggest that the total distance travelled through the plasma cannot be more than one or a few radiation lengths for example X0~10 m for 4x1022 e/cm3 using the rough estimate of 30 GV/m for 1x1017e/cm3 this gives an ultimate energy of ~200 TeV ...
High angle neutron-proton scattering
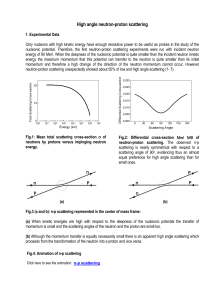
... neutron shell which is easily interchanged between protons. It presents some similitude with the Yukawa model but it differs in that instead of appealing to the exchange of a pion carrier it does to the exchange of the neutron shell with the impinging proton. The orbital model leads straightforwardl ...
... neutron shell which is easily interchanged between protons. It presents some similitude with the Yukawa model but it differs in that instead of appealing to the exchange of a pion carrier it does to the exchange of the neutron shell with the impinging proton. The orbital model leads straightforwardl ...
Chapter 7
... ejected electron increases linearly with the frequency of the radiation. • Interpretation for the photoelectric effect: right after an electron collides with a photon with sufficient energy, the electron is ejected out from the metal. • After the collision, the electron is ejected out immediately. ...
... ejected electron increases linearly with the frequency of the radiation. • Interpretation for the photoelectric effect: right after an electron collides with a photon with sufficient energy, the electron is ejected out from the metal. • After the collision, the electron is ejected out immediately. ...
Arrangement of Electrons in Atoms
... radiation has a dual wave-particle nature. ◦ Light exhibits both wavelike properties, but it can also be thought of as a stream of particles. ◦ Each particle carries a quantum or energy. ◦ These particles, called photons, is a particle of electromagnetic radiation having zero mass and carrying a qua ...
... radiation has a dual wave-particle nature. ◦ Light exhibits both wavelike properties, but it can also be thought of as a stream of particles. ◦ Each particle carries a quantum or energy. ◦ These particles, called photons, is a particle of electromagnetic radiation having zero mass and carrying a qua ...
Atomic physics
... will emit a photon of the difference in energy. However, if the excited atom has been previously ionized, in particular if one of its inner shell electrons has been removed, a phenomenon known as the Auger effect may take place where the quantity of energy is transferred to one of the bound electron ...
... will emit a photon of the difference in energy. However, if the excited atom has been previously ionized, in particular if one of its inner shell electrons has been removed, a phenomenon known as the Auger effect may take place where the quantity of energy is transferred to one of the bound electron ...
Fulltext
... In Figure 2a the high angle annular dark field (HAADF) image shows the HAADF STEM image of the QD ensemble and figure 2b shows for the first time by us the red light filtered CL image. There is a clear distribution of sizes present, see regions shown inside the triangles. The larger particles show C ...
... In Figure 2a the high angle annular dark field (HAADF) image shows the HAADF STEM image of the QD ensemble and figure 2b shows for the first time by us the red light filtered CL image. There is a clear distribution of sizes present, see regions shown inside the triangles. The larger particles show C ...
Modern IV - Wappingers Central School District
... can propagate through a vacuum. 4.3l Diffraction occurs when waves pass by obstacles or through openings. The wavelength of the incident wave and the size of the obstacle or opening affect how the wave spreads out. 4.3 Explain variations in wavelength and frequency in terms of the source of the vibr ...
... can propagate through a vacuum. 4.3l Diffraction occurs when waves pass by obstacles or through openings. The wavelength of the incident wave and the size of the obstacle or opening affect how the wave spreads out. 4.3 Explain variations in wavelength and frequency in terms of the source of the vibr ...
ENT145/3 Materials Engineering Tutorial 1 (Answer) 1. Why is it
... (b) Two important quantum-mechanical concepts associated with the Bohr model of the atom are (1) that electrons are particles moving in discrete orbitals, and (2) electron energy is quantized into shells. (c) Two important refinements resulting from the wave-mechanical atomic model are (1) that ele ...
... (b) Two important quantum-mechanical concepts associated with the Bohr model of the atom are (1) that electrons are particles moving in discrete orbitals, and (2) electron energy is quantized into shells. (c) Two important refinements resulting from the wave-mechanical atomic model are (1) that ele ...
Chapter 4 - Tolland High School
... • When light particles (photons) collide with a metal, the photons knock electrons (e-) loose • These electrons move toward the positive terminal creating an electric current (electricity) ...
... • When light particles (photons) collide with a metal, the photons knock electrons (e-) loose • These electrons move toward the positive terminal creating an electric current (electricity) ...
Chapter 9 - Lecture 1
... • Particle is not free • ∴ For acceptable ψ, boundary conditions must be set: • ψ must vanish at x = 0 and x = L ...
... • Particle is not free • ∴ For acceptable ψ, boundary conditions must be set: • ψ must vanish at x = 0 and x = L ...
QUANTUM NUMBERS
... are composed of several sub-orbitals. These rules seem strange, but trust me, they pop right out of the mathematics. The third quantum number is mL, the magnetic quantum number. It labels the sub-orbitals within a p, d or f orbital. The values of m depend on L. If L = 0, then m = 0; if L = 1, then m ...
... are composed of several sub-orbitals. These rules seem strange, but trust me, they pop right out of the mathematics. The third quantum number is mL, the magnetic quantum number. It labels the sub-orbitals within a p, d or f orbital. The values of m depend on L. If L = 0, then m = 0; if L = 1, then m ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.