of electrons - Midland ISD
... move. What does that tell us? • Would a magnet affect a light from a flashlight? (you could try this at home) • Probably not. • Therefore, the cathode ray must be a ...
... move. What does that tell us? • Would a magnet affect a light from a flashlight? (you could try this at home) • Probably not. • Therefore, the cathode ray must be a ...
Napoleon - Excellence Gateway
... What do we call a covalent bond where both of the shared pair of electrons come from the same atom? ...
... What do we call a covalent bond where both of the shared pair of electrons come from the same atom? ...
Class 39 1
... Pauli Exclusion Principle No two electrons can be in the same quantum state. In other words, no two electrons can possess the same set of four quantum numbers. The consequence of the Pauli Exclusion Principle is that when we fill the energy levels of an atom with electrons (where there are Z of them ...
... Pauli Exclusion Principle No two electrons can be in the same quantum state. In other words, no two electrons can possess the same set of four quantum numbers. The consequence of the Pauli Exclusion Principle is that when we fill the energy levels of an atom with electrons (where there are Z of them ...
Why Life Exists?
... the latest findings in quantum biology and biophysics have discovered that there is in fact a tremendous degree of coherence within all living systems. The accelerating electrons explain not only the Maxwell Equations and the Special Relativity, but the Heisenberg Uncertainty Relation, the Wave-Part ...
... the latest findings in quantum biology and biophysics have discovered that there is in fact a tremendous degree of coherence within all living systems. The accelerating electrons explain not only the Maxwell Equations and the Special Relativity, but the Heisenberg Uncertainty Relation, the Wave-Part ...
Radiation of an Electric Charge in a Screened Magnetic Monopole Potential Abstract
... parameter is small, the particles can get arbitrarily close (beyond precision we can achieve/maintain). This can be solved by extrapolating a more accurate value of F using the result of nearby larger impact parameters ( f actually behaves quite well in this area). Second issue that might be of inte ...
... parameter is small, the particles can get arbitrarily close (beyond precision we can achieve/maintain). This can be solved by extrapolating a more accurate value of F using the result of nearby larger impact parameters ( f actually behaves quite well in this area). Second issue that might be of inte ...
Worksheet 4 - Periodic Trends A number of physical and chemical
... However, not all electrons in an atom experience the same nuclear charge. Those closest to the nucleus experience the full nuclear charge and are held most strongly. As the number of electrons between the nucleus and the valence electrons increases, the apparent nuclear charge decreases, due to the ...
... However, not all electrons in an atom experience the same nuclear charge. Those closest to the nucleus experience the full nuclear charge and are held most strongly. As the number of electrons between the nucleus and the valence electrons increases, the apparent nuclear charge decreases, due to the ...
Lower-Hybrid Waves
... the ion temperature we utilize the fact that the ratio of ion and electron drifts is given by V T Di ...
... the ion temperature we utilize the fact that the ratio of ion and electron drifts is given by V T Di ...
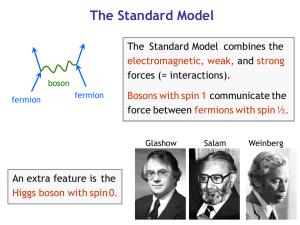
Bosons
... The anthropic principle The Standard Model does extremely well at predicting all kinds of measurements done by accelerators. But it is not able to calculate coupling constants. Some argue that coupling constants cannot be calculated: Different universes may have different coupling constants. Our un ...
... The anthropic principle The Standard Model does extremely well at predicting all kinds of measurements done by accelerators. But it is not able to calculate coupling constants. Some argue that coupling constants cannot be calculated: Different universes may have different coupling constants. Our un ...
if on the Internet, Press on your browser to
... "It's an oddball thing. But the mathematics is surprisingly simple to work out," says Goldberg. The new force acts as an extra attractive force, making Gravity appear a little bit stronger than you would expect for standard physics. Effects of the new force -- which Nath and Goldberg have dubbed "un ...
... "It's an oddball thing. But the mathematics is surprisingly simple to work out," says Goldberg. The new force acts as an extra attractive force, making Gravity appear a little bit stronger than you would expect for standard physics. Effects of the new force -- which Nath and Goldberg have dubbed "un ...
Atomic and Nuclear Physics
... higher one only if it receives an amount of energy equal to the difference in energy between the final and initial states. In this sense, Bohr’s model provided an explanation for the emission and absorption spectra ...
... higher one only if it receives an amount of energy equal to the difference in energy between the final and initial states. In this sense, Bohr’s model provided an explanation for the emission and absorption spectra ...
ppt
... 1. A kinematic threshold for the reaction p g e- e+ The photon at the proton rest frame must have energy greater than 2 me c2 . The synchrotron photon has an energy ES ~ bG2 at the plasma frame and ~ bG3 on the proton frame. So the condition reads ...
... 1. A kinematic threshold for the reaction p g e- e+ The photon at the proton rest frame must have energy greater than 2 me c2 . The synchrotron photon has an energy ES ~ bG2 at the plasma frame and ~ bG3 on the proton frame. So the condition reads ...
Chem 31 - Exam #3
... questions. For questions involving calculations, show all of your work -- HOW you arrived at a particular answer is MORE important than the answer itself! Circle your final answer to numerical questions. The entire exam is worth a total of 150 points. Attached are a periodic table and a formula shee ...
... questions. For questions involving calculations, show all of your work -- HOW you arrived at a particular answer is MORE important than the answer itself! Circle your final answer to numerical questions. The entire exam is worth a total of 150 points. Attached are a periodic table and a formula shee ...
IMFUFA- Roskilde Universitetscenter- postbox 260
... series is divergent. Formally, we can put z=-1, whereby the infinite sum becomes the previously mentioned sum of all positive integers, and we can assign it a value given by the analytical continuation of the zetafunction to z=-1. In this way we get at the renormalized value ζ(-1) = -1/124 , i.e., n ...
... series is divergent. Formally, we can put z=-1, whereby the infinite sum becomes the previously mentioned sum of all positive integers, and we can assign it a value given by the analytical continuation of the zetafunction to z=-1. In this way we get at the renormalized value ζ(-1) = -1/124 , i.e., n ...
Name Date Period 21-2 Radioactive Decay Match the following
... All of the elements with more than are radioactive. ...
... All of the elements with more than are radioactive. ...
ultimate standardmodell Kopie
... Both, scattering experiments with metal foils and X-ray experiments for nearly all elements were able to introduce the atomic number Z of target atoms as the variable of the corresponding metal foils or of the corresponding elements, respectively. This supposition is not justified because 1. Rutherf ...
... Both, scattering experiments with metal foils and X-ray experiments for nearly all elements were able to introduce the atomic number Z of target atoms as the variable of the corresponding metal foils or of the corresponding elements, respectively. This supposition is not justified because 1. Rutherf ...
Notes on Electron Configurations
... levels and orbitals are “filled” filled in order of increasing energy in order of increasing energy Energy increases going down the periodic table from top to bottom ...
... levels and orbitals are “filled” filled in order of increasing energy in order of increasing energy Energy increases going down the periodic table from top to bottom ...
IOSR Journal of Applied Physics (IOSR-JAP)
... Nuclear Force is defined as the force exerted between numbers of nucleons. This force is attractive in nature and binds protons and neutrons in the nucleus together. Since the protons are of same positive charge they exert a repulsive force among them. Because of this attractive Nuclear Force, the t ...
... Nuclear Force is defined as the force exerted between numbers of nucleons. This force is attractive in nature and binds protons and neutrons in the nucleus together. Since the protons are of same positive charge they exert a repulsive force among them. Because of this attractive Nuclear Force, the t ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.